
��14�֣���0.8 mol I2(g)��1.2 mol H2(g)����ij1L�ܱ������У���һ���¶��·�����Ӧ��I2(g)��H2(g)  2HI(g)���ﵽƽ�⡣HI�����������ʱ��ı仯�������ʾ��
2HI(g)���ﵽƽ�⡣HI�����������ʱ��ı仯�������ʾ��
HI������� | 1min | 2min | 3min | 4min | 5min | 6min | 7min |
����I | 26% | 42% | 52% | 57% | 60% | 60% | 60% |
����II | 20% | 33% | 43% | 52% | 57% | 65% | 65% |
��1��������I����ƽ��ʱ������÷�Ӧ��ƽ�ⳣ��K��Ҫ���г�������̡�
��2��������I�ӿ�ʼ��Ӧ������ƽ��ʱ��H2�ķ�Ӧ����Ϊ____________��
��3��Ϊ�ﵽ����II�����ݣ����ڷ�Ӧ��ϵ���ܸı�IJ�����_______________��
��4���÷�Ӧ�ġ�H_____0����">"��"<"��"="��
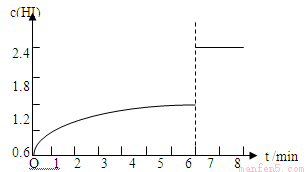
��5��������I�´ﵽƽ�����7minʱ���������ѹ��Ϊԭ����һ�롣����ͼ�л���c(HI)��ʱ��仯�����ߡ�

��1����I2����Ũ��Ϊx
I2(g) + H2(g)  2HI(g)
2HI(g)
��ʼŨ�ȣ�mol/L���� 0.8 1.2 0
ת��Ũ�ȣ�mol/L���� x x 2x
ƽ��Ũ�ȣ�mol/L����0.8-x 1.2-x 2x ��2�֣�
(�����е���ֵ��Ũ�Ȼ����ĵ�λʾ���1��)
HI���������Ϊ60%����2x/2=60%��x=0.6 mol/L ��1�֣�
K=c2 (HI) /[c(H2)��c(I2)]=1.22/(0.2��0.6)=12 ��1�֣�
��2��0.12 mol/(L��min) ��2�֣���λ����1�֣�
��3�������¶� (2��)
��4��< ��2�֣�
��5�� ��4�֣����θ�2�֣�
��������
�����������1�����ݻ�ѧƽ�����ƽ��ʱ�����ʵ�Ũ�ȣ���I2����Ũ��Ϊx
I2(g) + H2(g)  2HI(g)
2HI(g)
��ʼŨ�ȣ�mol/L���� 0.8 1.2 0
ת��Ũ�ȣ�mol/L���� x x 2x
ƽ��Ũ�ȣ�mol/L����0.8-x 1.2-x 2x
HI���������Ϊ60%����2x/2=60%��x=0.6 mol/L���ٸ���ƽ�ⳣ���ļ��㹫ʽK=c2 (HI) /[c(H2)��c(I2)]���м��㣬K=1.22/(0.2��0.6)=12����2�������ķ�Ӧ����=0.6/5=0.12mol/(L��min)����3�����ݱ����������ͬʱ���ڣ��⻯�����������仯С��˵�����������¶ȵ͡���4��ƽ��ʱ�⻯������������˵������ƽ�������ƶ�����H <0����5�� ���ѹ����ԭ��һ�룬��⻯���Ũ�ȱ��ԭ��2����ƽ�ⲻ�ƶ�������ͼ��Ϊ��

���㣺��ѧƽ��״̬�ļ�����ж���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015�㶫ʡ�����и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ʵĴ��������������
A�������Ʊ�����ʯ���ͻ�ú����
B�������ƻ�������������Ũ���ᡢŨ����
C��FeCl2��Һ���Լ�ƿ��Ҫ������
D������������Һʢװ�ڲ��������Լ�ƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015�㶫ʡ�����и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й���Ԫ�صȵ������У��������
A���������۵����100��
B����Ԫ��ֻ���Ի���̬��������Ȼ��
C��Na��Na��������ǿ�Ļ�ԭ��
D�������ƾ��кõĵ����ԣ����ճ��в���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015�㶫ʡ��������У�߶���ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ѡ����
ij��ɫ��Һ�������ܴ������棬ͨ��CO2�����ܴ��������һ����
A��K+��SO42-��Br-��SiO32- B��H+��Fe2+��Cl- ��NH4+
C��Na+��Ba2+��NO3-��Cl- D��Na+��Ag+��NH3H2O��NO3-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015�㶫ʡ��������У�߶���ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ѡ����
����������ȷ����
A��ǿ�������Һ�ĵ����Բ�һ�������������Һ�ĵ�����ǿ
B�������£���pH=3�Ĵ�����Һϡ�͵�ԭ�����10������Һ��pH=4
C���������ˮ��pHС��7�����µ�������
D����ͨ����н����ж�����ʵ����ϡ���ɫ��ѧ��˼��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015�㶫ʡ���ŵ���У�߶���ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ѡ����
����������ȷ����
A��KSp[MgF2]>KSp[Mg(OH)2]������ʵ��Mg(OH)2ת��ΪMgF2��
B�������£�ͬŨ�ȵ�Na2S��NaHS��Һ��ȣ�Na2S��Һ��pH��
C�������ʵ���Ũ�ȵ�NH4Cl��Һ��NH4HSO4��Һ�����ߵ�c(NH4��)��
D��FeCl3��KSCN��Ӧ�ﵽƽ��ʱ������KCl��Һ������Һ��ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015�㶫ʡ���ŵ���У�߶���ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ѡ����
����Ƭ��ϡ���ᷴӦ��ȡ����ʱ�����д�ʩ����ʹ�����������ʼӴ����
A������ B������ϡ���ᣬ����98%Ũ����
C���μ�����CuSO4��Һ D��������Ƭ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ������ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����±��ṩ�IJ�������(�Dz���������ѡ)����ʵ����Ӧʵ��Ŀ�ĵ���
ѡ�� | ʵ��Ŀ�� | �������� |
A | �����Ҵ������������Ļ���� | ��Һ©�����ձ� |
B | ��pH=1����������100 mL,pH=2������ | 100 mL����ƿ���ձ�������������ͷ�ι� |
C | ����ˮ������KI��Һ�Ƚ�Br2��I2��������ǿ�� | �Թܡ���ͷ�ι� |
D | ��NH4Cl�����Ca(OH)2�����Ʊ����ռ�NH3 | �ƾ��ơ��ձ������ܡ�����ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�����и�һ��ѧ�����п��Ի�ѧ���������棩 ���ͣ�ѡ����
����˵������ȷ����
A��1molNaOH��������40g B��1molCH4���ԼΪ22.4L
C��CO2��Ħ������Ϊ44g D��1molH2O��Լ��6.02��1023��H
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com