
 ��֪ A��B��C��D��ԭ������������������ֶ���������Ԫ�أ�A������������������������Bԭ�ӵļ۵����Ų�Ϊnsnnpn��D�ǵؿ��к�������Ԫ�أ�E�ǵ������ڵ�p��Ԫ���������ֻ��2�ԳɶԵ��ӣ�FԪ��ԭ������Ϊ29��
��֪ A��B��C��D��ԭ������������������ֶ���������Ԫ�أ�A������������������������Bԭ�ӵļ۵����Ų�Ϊnsnnpn��D�ǵؿ��к�������Ԫ�أ�E�ǵ������ڵ�p��Ԫ���������ֻ��2�ԳɶԵ��ӣ�FԪ��ԭ������Ϊ29��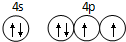 ��
������ A��������������������������AΪHԪ�أ�Bԭ�ӵļ۵����Ų�Ϊnsnnpn��n=2����BΪCԪ�أ�D�ǵؿ��к�������Ԫ�أ���DΪOԪ�أ�C��ԭ����������̼����֮�䣬��CΪNԪ�أ�E�ǵ������ڵ�p��Ԫ���������ֻ��2�ԳɶԵ��ӣ���Χ�����Ų�Ϊ4s24p4����EΪSeԪ�أ�FԪ��ԭ������Ϊ29����FΪCu���ݴ˽��
��� �⣺A��������������������������AΪHԪ�أ�Bԭ�ӵļ۵����Ų�Ϊnsnnpn��n=2����BΪCԪ�أ�D�ǵؿ��к�������Ԫ�أ���DΪOԪ�أ�C��ԭ����������̼����֮�䣬��CΪNԪ�أ�E�ǵ������ڵ�p��Ԫ���������ֻ��2�ԳɶԵ��ӣ���Χ�����Ų�Ϊ4s24p4����EΪSeԪ�أ�FԪ��ԭ������Ϊ29����FΪCu��
��1��E�ǵ������ڵ�p��Ԫ���������ֻ��2�ԳɶԵ��ӣ���Χ�����Ų�Ϊ4s24p4���۵����Ų�ͼΪ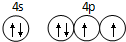 ���ʴ�Ϊ��
���ʴ�Ϊ��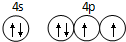 ��
��
��2��ͬ����Ԫ�ش�����Ԫ�صĵ�һ�����ܳ�������������Oԭ�Ӻ���۵����Ų�ʽ2s22p4��Nԭ�Ӻ���۵����Ų�ʽ2s22p3��2p�Dz����ڰ�������ȶ��ṹ����ʧȥһ�����ӣ���һ�����ܴ���Ԫ�أ���˳��ΪN��O��C���ʴ�Ϊ��N��O��C��
��3��CO32-����ԭ���γ�3���Ҽ����µ��Ӷ���Ϊ$\frac{6-2��3}{2}$=0���ӻ����������Ϊsp2��NH4+����ԭ���γ�4���Ҽ����ռ乹��Ϊ�������壬
�ʴ�Ϊ��sp2���������壻
��4��CN-��N2Ϊ�ȵ����壬����C��N������1mol BC-�к��Цм�����ĿΪ2NA���ʴ�Ϊ��2��
��5���ǽ�����O��S��Ԫ�صķǽ�����Խǿ������Խ��Ӧ���⻯��Խ�ȶ�����H2O��H2Se���ʴ�Ϊ��H2O��H2Se��
��6��CuԪ�صĻ�̬ԭ�Ӽ۵����Ų�ʽΪ3d104s1���ʴ�Ϊ��3d104s1��
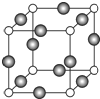
��7���ɾ����ṹ��֪��Nԭ��λ�ڶ��㣬������N����Ϊ8��$\frac{1}{8}$=1��Cuλ�����ϣ�������Cuԭ�Ӹ���Ϊ12��$\frac{1}{4}$=3����ѧʽΪCu3N��������λ�ڶ�������ĵ�λ���������Nԭ�ӵ���λ����6��
������Cԭ�Ӻ�Fԭ�Ӽ�ľ���Ϊa cm����߳�Ϊ2acm�����������Ϊ8a3cm3������������Ϊ$\frac{206}{{N}_{A}}$g�����ܶ�Ϊ$\frac{206}{{N}_{A}}$g��8a3cm3=$\frac{103}{4{a}^{3}{N}_{A}}$g/cm3��
�ʴ�Ϊ��Cu3N�� 6��$\frac{103}{4{a}^{3}{N}_{A}}$��
���� ���⿼�����ʵĽṹ�����ʣ��漰��������Ų��������ܡ��ӻ���ʽ���ռ乹�͡���ѧ����Ԫ��������Ӧ�á���������ȣ��Ѷ��еȣ�ע���������þ�̯�����о������йؼ��㣮


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ���� | HA���ʵ���Ũ�ȣ�mol•L1�� | NaOH���ʵ���Ũ�ȣ�mol•L��1�� | �����Һ��pH |
| �� | 0.2 | 0.2 | a |
| �� | c1 | 0.2 | 7 |
| �� | 0.1 | 0.1 | 7 |
| �� | 0.1 | 0.1 | 9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
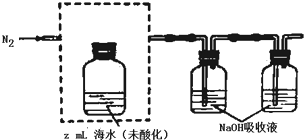
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 molAl3+���Ӻ��еĺ��������Ϊ3NA | |
| B�� | 1.7gH2O2�к��еĵ�����Ϊ0.9NA | |
| C�� | �����£�11.2L�ļ������庬�м��������Ϊ0.5NA�� | |
| D�� | ��״���£�33.6LH2O����9.03��1023��H2O���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SiO2������ˮ��Ҳ��������ͼ� | B�� | SiO2������̫���ܵ�صij��ò��� | ||
| C�� | ���ǵؿ��к������ķǽ���Ԫ�� | D�� | ˮ����������ľ�ķ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ú | B�� | ���� | C�� | ʯ������Ʒ | D�� | �ƾ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com