
(1)���ԣ�H2CO3______H2SiO3,H2SiO3______H3PO4
(2)���ԣ�Ca(OH)2______Mg(OH)2,Mg(OH)2______Al(OH)3
(3)��̬�⻯���ȶ��ԣ�H2O______H2S��H2S______HCl
(4)��ԭ�ԣ�H2O______H2S��H2S______HCl
(5)���ԣ�H2SO4______H2SO3��HClO4______HClO
�����ϴ��п��Թ��ɳ���
��Ԫ�صķǽ�����Խǿ�����Ӧ����������ˮ���������Խ_____��
��Ԫ�صĽ�����Խǿ�����Ӧ����������ˮ����ļ���Խ_____��
��Ԫ�ص���Խǿ�����Ӧ��̬�⻯����ȶ���Խ_____��
�ܷǽ�����Խǿ��Ԫ�����ɵ���̬�⻯��仹ԭ��Խ_____��
��ͬ�ַǽ���Ԫ���γɵĺ����ᣬ�����Ԫ�ؼ�̬Խ�ߣ�������ҲԽ_____��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ı����� | ����ƽ���ƶ� ���� |
����� | c��H+�� | c��CH3COO-�� |
| ��ˮϡ�� | ||||
| ������HCl��Һ | ||||
| ��������CH3COONa���� |
| c(NH4+)��c(OH-) |
| c(NH3��H2O) |
| c(NH4+)��c(OH-) |
| c(NH3��H2O) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
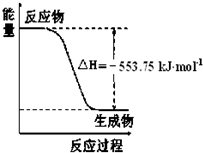 ��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵���
��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ı����� | ����ƽ���ƶ����� | c��H+�� | c��CH3COO-�� |
| ��ˮϡ�� | |||
| ������������ | |||
| ��������CH3COONa���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com