
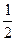 O2(g)
O2(g) ZnO(s);��H="-351.5" kJ��mol-1
ZnO(s);��H="-351.5" kJ��mol-1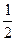 O2(g)
O2(g)  HgO(s);��H
HgO(s);��H  ="-90.84" kJ��mol-1
="-90.84" kJ��mol-1 ZnO(s)+Hg(l)�Ħ�HΪ
ZnO(s)+Hg(l)�Ħ�HΪ| A����H="+260.7" kJ��mol-1 | B����H="-260.7" kJ��mol-1 |
| C����H="-444.2" kJ��mol-1 | D����H="+444.2" kJ��mol-1 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����2H2��g��+O2��g��=2H2O��l����H= ��571.6 kJ/mol ��֪������ȼ���ȡ�H= ��285.8 kJ/mol |
| B��ij���ӱ�������ȫ��ָ����������Һ�е�Ũ��Ϊ0 |
| C����⾫��ͭʱ�Դ�ͭ������ |
| D������һ�������Ŀ��淴Ӧ��ƽ�ⳣ��ֻ���¶��йأ���Ũ�ȡ�ѹǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2SO3 (g)������H =��196.6kJ��mol��1
2SO3 (g)������H =��196.6kJ��mol��1 2NO2 (g)������H =��113.0kJ��mol��1
2NO2 (g)������H =��113.0kJ��mol��1 SO3 (g) +NO(g)�ġ�H =______kJ��mol��1
SO3 (g) +NO(g)�ġ�H =______kJ��mol��1 CH3OH (g)��CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ����ͼ��ʾ���÷�Ӧ��H _____0���������������ʵ����������������250�桢1.3��104kPa���ң�
CH3OH (g)��CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ����ͼ��ʾ���÷�Ӧ��H _____0���������������ʵ����������������250�桢1.3��104kPa���ң�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��+488.3kJ/mol | B����488.3 kJ/mol |
| C����244.15 kJ/mol | D��+244.15 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ϡ��Һ�����кͷ�Ӧ���к���Ϊ57.3 kJ/mol�������ȼ����Ϊ5518 kJ/mol�������Ȼ�ѧ����ʽ��д��ȷ���ǣ� ��
ϡ��Һ�����кͷ�Ӧ���к���Ϊ57.3 kJ/mol�������ȼ����Ϊ5518 kJ/mol�������Ȼ�ѧ����ʽ��д��ȷ���ǣ� ��| A��2H+(aq) +SO42��(aq)+Ba2+(aq)+2OH��(aq)=BaSO4(s)+2H2O(1)����H=��57.3 kJ/mol |
B��KOH(aq )+ )+ H2 SO4(aq)=K2SO4(aq)+H2O(l)����H=��57.3kJ/mol H2 SO4(aq)=K2SO4(aq)+H2O(l)����H=��57.3kJ/mol |
| C��2C8H18(g)+25O2(g)=16CO2(g)+18H2O(1)����H=��5518 kJ/mol |
| D��C8H18(l)+ 25/2 O2(g)=8CO2(g)+ 9H2O(1)����H=��5518 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���缫����ʽ��Cu-2e- = Cu2+�����ܷ�����ԭ����У����ܷ����ڵ����� |
| B��ij�¶��£�10mL 0.1mol/L��H2SO4��Һ��10mL 0.4 mol/L��KOH��Һ��Ϻ�pH=13 |
| C������1mol H-H��N-H��N��N�������յ������ֱ�Ϊa kJ��b kJ��c kJ�� ��2NH3( g) 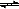 3H2(g)��N2(g) ��H="(3a+c-6b)" kJ/mol 3H2(g)��N2(g) ��H="(3a+c-6b)" kJ/mol |
| D���к͵�����Ĵ�����Һ�����ĵ�pHֵ�İ�ˮ������������Һ������ֱ�ΪV1��V2����V1<V2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��488.3 kJ��mol��1������������ | B����244.15 kJ��mol��1 |
| C��244.15 kJ��mol��1 | D����488.3 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 4������a mol CH4��CO��H2���������ȫȼ�գ�����CO2�����Һ̬ˮ����CO2��H2O��
4������a mol CH4��CO��H2���������ȫȼ�գ�����CO2�����Һ̬ˮ����CO2��H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com