
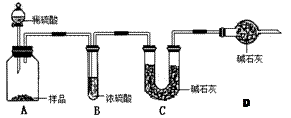
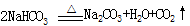 ����ش�
����ش�| ��������� ���� | ������Ʒ��̼���Ƶ��������� | ʵ���������� | ʵ��ʧ�ܵ���Ҫԭ�Խ����Ӱ�� |
| �� | | ʧ �� | |
| �� | |  �� �� �� �� | |
| �� | |  �� �� �� �� | |
| ������Ʒ��̼���Ƶ��������� | ʵ��ʧ�ܵ���Ҫԭ�Խ����Ӱ�� |
| 84.8% | ��Ӧ���ɵ�CO2���ֲ�����ƿA�У�û�б���ȫ���գ����ƫС |
| 95.4% | |
| 95.4% | |
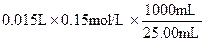 ��0.09mol��Na2CO3��������0.09mol��106g/mol��9.54g����������Ϊ9.54%��92%<9.54%<96%��������Χ���������У���10.00g ��Ʒ�к���Na2CO3������Ϊx g�����ݷ�Ӧ��Na2CO3�� 2HCl ��2NaCl + CO2�� + H2O�����õ�NaCl��������
��0.09mol��Na2CO3��������0.09mol��106g/mol��9.54g����������Ϊ9.54%��92%<9.54%<96%��������Χ���������У���10.00g ��Ʒ�к���Na2CO3������Ϊx g�����ݷ�Ӧ��Na2CO3�� 2HCl ��2NaCl + CO2�� + H2O�����õ�NaCl��������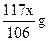 �����У�10.00��x ��
�����У�10.00��x ��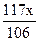 ��10.99�����x��9.54g��Na2CO3��������Ϊ9.54%��92%<9.54%<96%��������Χ��
��10.99�����x��9.54g��Na2CO3��������Ϊ9.54%��92%<9.54%<96%��������Χ��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
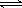 H++HCO3- Ka1 =4��45��10-7
H++HCO3- Ka1 =4��45��10-7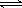 H++CO32- Ka2=5��61��10-11
H++CO32- Ka2=5��61��10-11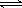 H++ClO- Ka=2��95��10-8
H++ClO- Ka=2��95��10-8�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
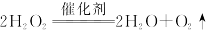
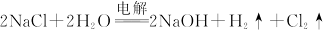
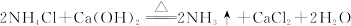
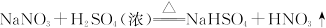
| A���٢ڢۢ� |
| B���� |
| C���ڢ� |
| D��ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ֳ�ɫ�㷨��ɫ�������Դ����ɫ���ʵķ��� |
| B������������ɫ����û�з������� |
| C���÷۱�Ҳ���Զ�Ҷ����a��Ҷ����b�������ϲ�����ԭ����ֽ�ϲ���һ�� |
| D��������Ҫ��������Ա����������к��ʵ��ܽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
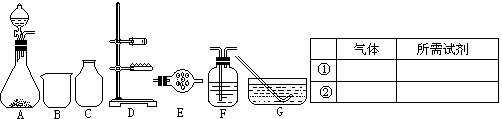
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
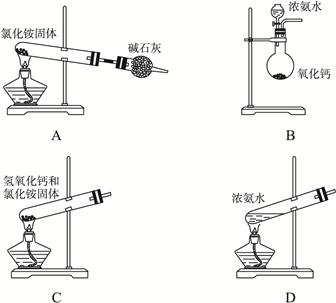
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
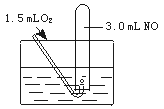
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
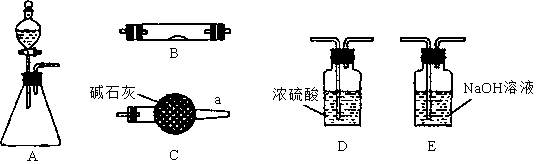
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��



| A��ʵ������ȡ��������������ʱ��Ũ��������������ˮ�� |
| B����ͭ˿���Ⱥ����뵽�Ҵ��У���Ӧ��ͭ˿���������� |
| C���ñ���ȡ��ˮ�е��壬��Һʱ�л���ӷ�Һ©�����Ͽڵ��� |
| D������ȡ��������������ʱ�������Թ��м���Ũ���ᣬ�ټ����Ҵ�������Ļ��Һ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com