
ЎѕМвДїЎїКµСйКТТЄЕдЦЖ480 mL 0.2 mol/L HClИЬТєЈ¬Зл»ШґрПВБРОКМвЈє
(1)ЕдЦЖИЬТєК±µДІЅЦиУРЈєўЩјЖЛг ўЪИЬЅв ўЫПґµУ ўЬ(»ЦёґКТОВєу)ТЖТє ўЭ¶ЁИЭ ўЮТЎФИ ўЯіЖБїЈ¬ХэИ·µДЛіРтКЗ___________ЎЈ
A. ўЩўЪўЫўЬўЭўЮўЯ B. ўЩўЯўЪўЬўЫўЭўЮ C. ўЩўЯўЪўЬўЫўЮўЭ
(2)ЕдЦЖ№эіМЦРІ»РиТЄК№УГµД»ЇС§ТЗЖчУР____________(МоСЎПоµДЧЦДё)ЎЈ
A. ЙХ± B. 500 mLИЭБїЖї C. В©¶· D. ЅєН·µО№Ь E. ІЈБ§°ф
(3)ґУЛщЕдHClИЬТєЦРИЎіц10 mLИЬТєЈ¬УлФИЬТєПа±ИЈ¬»№»б·ўЙъ±д»ЇµДКЗ_________ЎЈ
A. ИЬТєЦРHClµДОпЦКµДБї B. ИЬТєЦРHClµДОпЦКµДБїЕЁ¶И
C. ИЬТєЦРCl-µДКэДї D. HClµДЦКБї
(4)ФЪЕдЦЖЙПКцИЬТєµД№эіМЦРЈ¬ПВБРЗйїц¶ФHClИЬТєОпЦКµДБїЕЁ¶ИУРєОУ°Пм(МоЎ°Ж«ёЯЎ±ЎўЎ°Ж«µНЎ±Ўў»тЎ°ОЮУ°ПмЎ±)
ўЩЧЄТЖИЬТєєуЈ¬ОґПґµУЙХ±єНІЈБ§°фѕНЦ±ЅУ¶ЁИЭ___________ЎЈ
ўЪИЭБїЖїУГХфБуПґµУєуІРБфУРЙЩБїµДЛ®Јє___________ЎЈ
ўЫ¶ЁИЭК±Ј¬Л®ОґјУµЅїМ¶И___________ЎЈ
(5)Из№ы¶ЁИЭК±јУЛ®і¬№эИЭБїЖїµДїМ¶ИПЯЈ¬РиТЄІЙИЎґлК©КЗ__________ЎЈ
Ўѕґр°ёЎїB C ACD Ж«µН ОЮУ°Пм Ж«ёЯ µ№µфЦШРВЕдЦЖ
ЎѕЅвОцЎї
(1)ёщѕЭЕдЦЖТ»¶ЁМе»эЎўТ»¶ЁОпЦКµДБїЕЁ¶ИИЬТєµДІЅЦиЕЕРтЈ»
(2)ТАѕЭЕдЦЖИЬТєМе»эСЎФсРиТЄИЭБїЖї№жёсЈ¬ТАѕЭЕдЦЖТ»¶ЁОпЦКµДБїЕЁ¶ИИЬТєµДТ»°гІЅЦиСЎФсРиТЄµДТЗЖчЈ»
(3)ёщѕЭИЬТєѕЯУРѕщТ»РФЈ¬УлИЬТєµДМе»эОЮ№ШЈ¬ЅбєПn=cЎ¤Vј°m=nЎ¤M·ЦОцЕР¶ПЈ»
(4)ёщѕЭИЬТєµДОпЦКµДБїЕЁ¶И¶ЁТеКЅc=![]() ·ЦОцКµСйОуІоЈ»
·ЦОцКµСйОуІоЈ»
(5)ёщѕЭИЭБїЖїКЗЧјИ·ЕдЦЖТ»¶ЁОпЦКµДБїЕЁ¶ИµДИЬТєµДТЗЖчИ·¶ЁКµСйІЩЧч·Ѕ·ЁЎЈ
(1)ФЪКµСйКТЦРЕдЦЖТ»¶ЁМе»эТ»¶ЁОпЦКµДБїЕЁ¶ИИЬТєµДІЅЦиКЗјЖЛгЎўіЖБї(»тБїИЎ)ЎўИЬЅв(»тПЎКН)ЎўАдИґЎўТЖТєЎўПґµУЎў¶ЁИЭЎўТЎФИЈ¬ТтґЛІЩЧчЛіРтУГРтєЕ±нКѕОЄўЩўЯўЪўЬўЫўЭўЮЈ¬єПАнСЎПоКЗBЈ»
(2)ЕдЦЖТ»¶ЁОпЦКµДБїЕЁ¶ИИЬТєТЄК№УГТ»¶Ё№жёсµДИЭБїЖїЈ¬СЎФсТЗЖчµД±кЧјКЗЎ°ґу¶шЅьЎ±Ј¬№КТЄК№УГ500 mLµДИЭБїЖїЈ¬ЕдЦЖИЬТєµДТ»°гІЅЦиЈєјЖЛгЎўіЖБї(»тБїИЎ)ЎўИЬЅв(»тПЎКН)ЎўАдИґЎўТЖТєЎўПґµУЎў¶ЁИЭЎўТЎФИЎЈЕдЦЖСОЛбЈ¬ТЄК№УГБїНІБїИЎЕЁСОЛбЈ¬И»єуФЪЙХ±ЦРЅшРРПЎКНЈ¬УГІЈБ§°фЅБ°иґЩЅшОпЦКµДИЬЅвЈ¬ФЪПтИЭБїЖїЦРТЖТєК±ТЄК№УГІЈБ§°фТэБчЈ¬ФЪЧоєу¶ЁИЭК±ТЄК№УГЅєН·µО№ЬЈ¬№КІ»К№УГµДТЗЖчКЗВ©¶·Ј¬№КєПАнСЎПоКЗCЈ»
(3)ИЬТєµДЕЁ¶ИУлЛщИЎИЬТєµДМе»эОЮ№ШЈ¬ЛщТФґУЛщЕдHClИЬТєЦРИЎіц10 mLИЬТєЈ¬ИЬТєµДЕЁ¶ИІ»±дЈ¬µ«УЙУЪИЬТєµДМе»эјхРЎЈ¬ёщѕЭОпЦКµДБїЕЁ¶И¶ЁТеКЅїЙЦЄn=cЎ¤VЈ¬ИЬТєМе»эјхРЎЈ¬ФтИЬЦКHClµДОпЦКµДБїјхЙЩЈ¬ёГИЬТєЦРµзАлІъЙъµДCl-µДКэДїТІјхРЎЈ»ёщѕЭm=nЎ¤MїЙЦЄЈ¬ИЬЦКµДОпЦКµДБїјхЙЩЈ¬ФтёГИЬТєЦРє¬УРµДИЬЦКµДЦКБїТІјхРЎЈ¬№К·ўЙъ±д»ЇµДСЎПоКЗACDЈ»
(4)ўЩЧЄТЖИЬТєєуЈ¬ОґПґµУЙХ±єНІЈБ§°фѕНЦ±ЅУ¶ЁИЭЈ¬»бµјЦВИЬЦКµДОпЦКµДјхЙЩЈ¬ёщѕЭЕЁ¶И¶ЁТеКЅїЙЦЄЈ¬ИЬЦКµДОпЦКµДБїјхРЎЈ¬µ«ИЬТєМе»эІ»±дЈ¬ЧоЦХК№ЕдЦЖµДИЬТєЕЁ¶ИЖ«µНЈ»
ўЪИЭБїЖїУГХфБуПґµУєуІРБфУРЙЩБїµДЛ®Ј¬УЙУЪІ»У°ПмИЬЦКµДОпЦКµДБїј°ЧоєуИЬТєµДМе»эЈ¬ТтґЛ¶ФЕдЦЖИЬТєµДЕЁ¶ИОЮУ°ПмЈ»
ўЫ¶ЁИЭК±Ј¬Л®ОґјУµЅїМ¶ИЈ¬ФтИЬТєµДМе»эЖ«РЎЈ¬УЙУЪИЬЦКµДОпЦКµДБїІ»±дЈ¬ТтґЛЧоЦХµјЦВЕдЦЖИЬТєµДЕЁ¶ИЖ«ёЯЈ»
(5)Из№ы¶ЁИЭК±јУЛ®і¬№эИЭБїЖїµДїМ¶ИПЯЈ¬К№ИЬТєµДМе»эЖ«ґуЈ¬µјЦВИЬТєµДЕЁ¶ИЖ«µНЈ¬РиТЄІЙИЎґлК©КЗЅ«ёГИЬТєµ№µфЈ¬ЦШРВЕдЦЖИЬТєЎЈ


 їЪЛгРДЛгЛЩЛгУ¦УГМвПµБРґр°ё
їЪЛгРДЛгЛЩЛгУ¦УГМвПµБРґр°ё Н¬ІЅНШХ№ФД¶БПµБРґр°ё
Н¬ІЅНШХ№ФД¶БПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїТСЦЄДі±ҐєНИЬТєµДЈєўЩИЬјБµДЦКБїЈ»ўЪИЬТєµДЦКБїЈ»ўЫИЬТєµДМе»эЈ»ўЬИЬЦКµДД¦¶ыЦКБїЈ»ўЭИЬЦКµДИЬЅв¶ИЈ»ўЮИЬТєµДГЬ¶ИЎЈПВБРЧйєПІ»ДЬјЖЛгіцёГИЬТєµДОпЦКµДБїЕЁ¶ИµДКЗ
A. ўЩўЪўЬўЮ B. ўЬўЭўЮ C. ўЪўЫўЬўЭ D. ўЫўЬўЮ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїіЈОВПВЈ¬ПВБРёчЧйАлЧУФЪЦё¶ЁИЬТєЦРДЬґуБї№ІґжµДКЗ
A.pHЈЅ1µДИЬТєЦРЈєKЈ«ЎўFe2Ј«ЎўMnO![]() ЎўSO
ЎўSO![]()
B.c(Fe3Ј«)ЈЅ0.1 molЎ¤LЈ1µДИЬТєЦРЈєKЈ«ЎўClOЈЎўSO![]() ЎўSCNЈ
ЎўSCNЈ
C.c(HЈ«)/c(OHЈ)ЈЅ1012µДИЬТєЦРЈєNH![]() ЎўAl3Ј«ЎўNO
ЎўAl3Ј«ЎўNO![]() ЎўClЈ
ЎўClЈ
D.УлВБ·ґУ¦ЙъіЙЗвЖшµДИЬТєЦРЈєNH![]() ЎўKЈ«ЎўClЈЎўSiO
ЎўKЈ«ЎўClЈЎўSiO![]()
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїФЪєгИЭГЬ±ХИЭЖчЦРНЁИлXІў·ўЙъ·ґУ¦Јє2X(g)![]() Y(g)Ј¬ОВ¶ИT1ЎўT2ПВXµДОпЦКµДБїЕЁ¶Иc(X)ЛжК±јдt±д»ЇµДЗъПЯИзНјЛщКѕЈ¬ПВБРРрКцХэИ·µДКЗЈЁ Ј©
Y(g)Ј¬ОВ¶ИT1ЎўT2ПВXµДОпЦКµДБїЕЁ¶Иc(X)ЛжК±јдt±д»ЇµДЗъПЯИзНјЛщКѕЈ¬ПВБРРрКцХэИ·µДКЗЈЁ Ј©
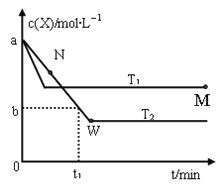
A. ёГ·ґУ¦ЅшРРµЅMµг·ЕіцµДИИБїґуУЪЅшРРµЅWµг·ЕіцµДИИБї
B. T2ПВЈ¬ФЪ0--t1К±јдДЪЈ¬v(Y)=![]()
C. MµгµДХэ·ґУ¦ЛЩВКРЎУЪNµгµДДж·ґУ¦ЛЩВК
D. MµгК±ФЩјУИлТ»¶ЁБїµДXЈ¬ЖЅєвєуXµДЧЄ»ЇВКФцґу
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїТСЦЄУРТФПВОпЦКП໥ת»ЇЈє
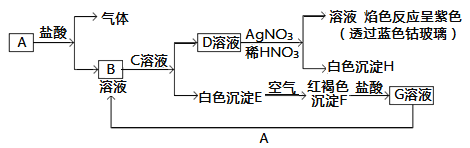
КФ»ШґрЈє
(1)РґіцBµД»ЇС§КЅ________Ј¬DµД»ЇС§КЅ________ЎЈ
(2)РґіцУЙEЧЄ±діЙFµД»ЇС§·ЅіМКЅ________________________ЎЈ
(3)РґіцУГKSCNјш±рGИЬТєµДАлЧУ·ЅіМКЅ________________Ј»ПтGИЬТєјУИлAµДУР№ШАлЧУ·ґУ¦·ЅіМКЅ________________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїТСЦЄјЧЎўТТЎў±ыИэЦЦОпЦКѕщє¬УРН¬ЦЦФЄЛШXЈ¬ЖдЧЄ»Ї№ШПµИзПВЈє
![]()
ПВБРЛµ·ЁґнОуµДКЗ
A.ИфAОЄNaOHИЬТєЈ¬ТТОЄ°ЧЙ«іБµнЈ¬ФтXїЙДЬОЄ¶МЦЬЖЪЅрКфФЄЛШ
B.ИфAОЄПхЛбЈ¬XОЄЅрКфФЄЛШЈ¬ФтјЧУлТТ·ґУ¦їЙЙъіЙ±ы
C.ИфAОЄСхЖшЈ¬±ыФЪНЁіЈЧґїцПВОЄємЧШЙ«ЖшМеЈ¬ФтјЧїЙДЬОЄ·ЗЅрКфµҐЦК
D.ИфТТОЄNaHCO3Ј¬ФтјЧ»т±ыїЙДЬКЗCO2
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїµюµЄ»ЇДЖ(NaN3)КЗТ»ЦЦТЧИЬУЪЛ®µД°ЧЙ«ѕ§МеЈ¬ОўИЬУЪТТґјЈ¬І»ИЬУЪТТГСЈ¬їЙУГУЪєПіЙї№ЙъЛШН·жЯѕъЛШТ©ОпµДЦРјдМеЎўЖыіµ°ІИ«ЖшДТµДТ©јБµИЎЈ°±»щДЖ(NaNH2)µДИЫµгОЄ210ЎжЈ¬·РµгОЄ400ЎжЈ¬ФЪЛ®ИЬТєЦРТЧЛ®ЅвЎЈКµСйКТЦЖИЎµюµЄ»ЇДЖµДКµСйІЅЦиј°КµСйЧ°ЦГИзПВЈє
ўЩґтїЄЦ№Л®јРK1Ј¬№Ш±ХЦ№Л®јРK2Ј¬јУИИЧ°ЦГDТ»¶ОК±јдЈ»
ўЪјУИИЧ°ЦГAЦРµДЅрКфДЖЈ¬К№ЖдИЫ»ЇІўід·Ц·ґУ¦єуЈ¬ФЩНЈЦ№јУИИЧ°ЦГDІў№Ш±ХK1Ј»
ўЫПтЧ°ЦГAЦРbИЭЖчДЪјУИлјУИИЅйЦКІўјУИИµЅ210Ў«220ЎжЈ¬ґтїЄЦ№Л®јРK2Ј¬НЁИлN2OЈ»
ўЬАдИґЈ¬ПтІъОпЦРјУИлТТґјЈ¬јхС№ЕЁЛхЅбѕ§єуЈ¬ФЩ№эВЛЈ¬ІўУГТТГСПґµУЈ¬БАёЙЎЈ
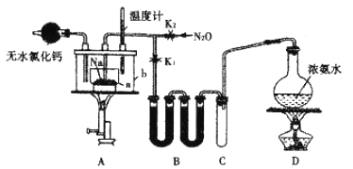
»ШґрПВБРОКМвЈє
(1)Ч°ЦГBЦРКў·ЕµДТ©Ж·ОЄ____________ЎЈ
(2)°±ЖшУлИЫ»ЇµДДЖ·ґУ¦ЙъіЙNaNH2µД»ЇС§·ЅіМКЅОЄ__________ЎЈ
(3)ІЅЦиўЫЦРЈ¬ОЄБЛК№·ґУ¦КЬИИѕщФИЈ¬AЧ°ЦГАпaИЭЖчµДјУИИ·ЅКЅОЄ__________Ј»ЙъіЙNaN3µД»ЇС§·ЅіМКЅОЄ__________Ј»N2OїЙУЙNH4NO3ФЪ240~245Ўж·ЦЅвЦЖµГ(ПхЛб淋ДИЫµгОЄ169.6Ўж)Ј¬ФтУ¦СЎФсµДЖшМе·ўЙъЧ°ЦГКЗ_____________(МоРтєЕ)ЎЈ

(4)ІЅЦиўЬЦРУГТТГСПґµУµДЦчТЄДїµДКЗ______________ЎЈ
(5)КµСйКТУГµО¶Ё·ЁІв¶ЁµюµЄ»ЇДЖСщЖ·ЦРNaN3µДЦКБї·ЦКэЈє
ўЩЅ«2.500gКФСщЕдіЙ500.00mLИЬТєЎЈ
ўЪИЎ50.00mLИЬТєЦГУЪЧ¶РОЖїЦРЈ¬јУИл50.00mL0.1010mol/L(NH4)2Ce(NO3)6ИЬТєЎЈ
ўЫід·Ц·ґУ¦єуЈ¬Ѕ«ИЬТєЙФПЎКНЈ¬ПтИЬТєЦРјУИл8mLЕЁБтЛбЈ¬µОИл3µОБЪ·Ж†ЄЯшЦёКѕТєЈ¬УГ0.0500mol/L(NH4)2Fe(SO4)2±кЧјИЬТєµО¶Ё№эБїµДCe4+ПыєДИЬТєМе»эОЄ29.00mLЎЈІв¶Ё№эіМµД·ґУ¦·ЅіМКЅОЄЈє2(NH4)2Ce(NO3)6+2NaN3=4NH4NO3+2Ce(NO3)3+2NaNO3+3N2ЎьЈ»Ce4++Fe2+=Ce3++Fe3+Ј¬ФтКФСщЦРNaN3µДЦКБї·ЦКэОЄ_______________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎї(1)ТТґј(C2H5OH)КЗОґАґДЪИј»ъµДКЧСЎ»·±ЈРНТєМеИјБПЈ¬ЛьїЙТФУЙВМЙ«ЦІОпµДЅХёСЦЖИЎЈ¬1.0gТТґјНкИ«ИјЙХЙъіЙТєМ¬Л®·Еіц1.367kJИИБїЈ¬±нКѕТТґјИјЙХИИµДИИ»ЇС§·ЅіМКЅ_____________ЎЈ
(2)¶ПїЄ1molAB(g)·ЦЧУЦРµД»ЇС§јьК№Жд·Ц±рЙъіЙЖшМ¬AФЧУєНЖшМ¬BФЧУЛщОьКХµДДЬБїіЖОЄAЎЄBјьµДјьДЬЎЈПВ±нБРіцБЛТ»Р©»ЇС§јьµДјьДЬEЈє
»ЇС§јь | H-H | O=O | O-H |
E/kJЎ¤mol-1 | 436 | x | 463 |
Зл»ШґрПВБРОКМвЈє
ўЩИзНј±нКѕДі·ґУ¦µДДЬБї±д»Ї№ШПµЈ¬ФтґЛ·ґУ¦ОЄ________(МоЎ°ОьИИЎ±»тЎ°·ЕИИЎ±)·ґУ¦Ј¬ЖдЦР¦¤HЈЅ__________(УГє¬УРaЎўbµД№ШПµКЅ±нКѕ)ЎЈ
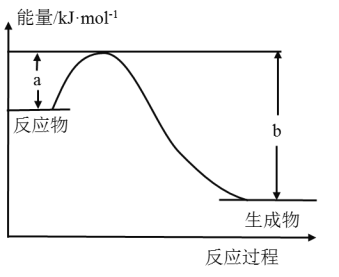
ўЪИфНјКѕЦР±нКѕ·ґУ¦H2(g)Ј«![]() O2(g)=H2O(g)¦¤HЈЅЈ241.8kJЎ¤molЈ1Ј¬ФтbЈЅ_______kJЎ¤molЈ1Ј¬xЈЅ________ЎЈ
O2(g)=H2O(g)¦¤HЈЅЈ241.8kJЎ¤molЈ1Ј¬ФтbЈЅ_______kJЎ¤molЈ1Ј¬xЈЅ________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїЅрКфТ±Б¶У봦АнЦРіЈЙжј°АлЧУ·ґУ¦єНСх»Ї»№Ф·ґУ¦ЎЈ
ЈЁ1Ј©КµСйКТіЈУГОЮЛ®ТТґјґ¦АнЙЩБїІРБфµДЅрКфДЖЈ¬Рґіц·ґУ¦µД»ЇС§·ЅіМКЅЈє________ЎЈ
ЈЁ2Ј©ГѕУлМјФЪјУЗїИИК±їЙЙъіЙТЧЛ®ЅвµДMgC2Ј¬ЖдЛ®Ѕв·ЅіМКЅОЄЈє________ЎЈ
ЈЁ3Ј©ОТ№ъ№ЕґъАН¶ЇИЛГсФшАыУГГч·ЇИЬТєіэИҐНЖч±нГжµДНРв[Cu2(OH)2CO3]Ј¬ЗлјтТЄЅвКНЖдФАнЈє________ЎЈ
ЈЁ4Ј©»ЖМъїу(FeS2)мСЙХК±їЙТФ·ўЙъИзПВ·ґУ¦Јє3FeS2+8O2![]() 6SO2+Fe3O4Ј¬µ±УР3mol SO2ЙъіЙК±Ј¬ЧЄТЖ________molµзЧУЎЈ
6SO2+Fe3O4Ј¬µ±УР3mol SO2ЙъіЙК±Ј¬ЧЄТЖ________molµзЧУЎЈ
Ійїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com