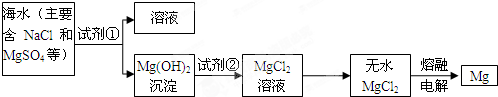
Ŀǰ������60%��þ�ǴӺ�ˮ��ȡ�ģ���ˮ��þ����Ҫ�������£�
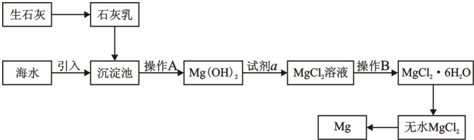
��ش��������⣺
��1�������ӷ�Ӧ�ĽǶ�˼�����ں�ˮ�м���ʯ�����������
����Mg2+����ʹMg2+�γ�Mg��OH��2������
����Mg2+����ʹMg2+�γ�Mg��OH��2������
��д���ڳ����ص����ӷ���ʽ
Mg2++2OH-�TMg��OH��2��
Mg2++2OH-�TMg��OH��2��
��
��2��ʯ��������ʯ����ˮ�γɵĻ�����ӳ�����ú���ѧ��Դ����߾���Ч��ĽǶȣ�������ʯ�ҵ���Ҫԭ����Դ�ں����е�
����
����
��
��3������A��
����
����
������B��
����Ũ��
����Ũ��
��
��4������������Լ�a��
HCl
HCl
���ѧʽ����
��5����ˮMgCl
2������״̬�£�ͨ�������Mg��Cl
2���÷�Ӧ�Ļ�ѧ����ʽΪ
���ӿ��dzɱ��ͷ���ѭ�����õĽǶȣ�������������������
�����ᣬѭ��ʹ��
�����ᣬѭ��ʹ��
��
��6����ˮ��þ�Ĺ��̣�ΪʲôҪ����ˮ�е��Ȼ�þת��Ϊ������þ����ת��Ϊ�Ȼ�þ��
��ˮ���Ȼ�þ�ĺ����ܴ�þ����Ũ�Ⱥܵͣ��ù��̿���ʹþ���Ӹ�����Ũ�ȸߣ�
�ҳɱ��ͣ�
��ˮ���Ȼ�þ�ĺ����ܴ�þ����Ũ�Ⱥܵͣ��ù��̿���ʹþ���Ӹ�����Ũ�ȸߣ�
�ҳɱ��ͣ�
��
��7����ͬѧ��Ϊ����ֱ�Ӽ���Mg��OH��
2�õ�MgO���ٵ������MgO�ƽ���þ�������ɼ�ʵ�鲽�裬����ʵ��ļ�Լ��ԭ����
��ͬ��
��ͬ��
���ͬ�⡱��ͬ�⡱����ͬѧ���뷨��������
MgO�۵�ܸߣ�����ʱ�ķѴ��������������������ɱ�
MgO�۵�ܸߣ�����ʱ�ķѴ��������������������ɱ�
��



 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�