
�й�ʮ�˴�������������ƽ���̬�������衱��
�� ȼú�����Ӵ���PM2.5��ֵ���γ������������������֮һ��ú�������Ǹ�Ч����������ú̿����Ҫ;����д�����ȵĽ�̿��ˮ������Ӧ�Ļ�ѧ����ʽ ��
�� ����ҵ�����ġ��ع��͡�����Ҫ�ɷ�����֬���ۺ����á��ع��͡���һ�ַ����ǽ����ع��͡��е���֬ˮ���Ի�ȡ��֬����� �������ƣ������������Ͻ��� ���������Ի����ϩ����ϩ�Ȼ���ԭ�ϡ�
�� �����ؽ�����Ⱦ��2013��ȫ�������������ص㡣����Hg2+�ķ�ˮ�м���Na2S������ʹHg2+ת��ɳ��������ӷ�Ӧ����ʽΪ ��
�� �ҹ���������һ��ɷ�Ϊ�����Ĵ��ࣺ�ɻ��������������������к����������������������������ڿɻ����������� ������ĸ����
a. �ϱ�ֽ b. ������������ c. ����ҩƷ d. ����
�� C + H2O 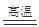 CO + H2
CO + H2
�ڸ��� �ѽ⣨�����ѽ� ��
�� Hg2+ + S2��= HgS��
�� a b
��������������ٽ�̿��ˮ������Ӧ�ɵ�ˮú��CO��H2����ѧ����ʽΪC + H2O  CO + H2 ��
CO + H2 ��
����֬�ijɷ��Ǹ�֬����ĸ�������ˮ��������֬������ͣ�������ϩ���ľۺ�����������Ͻ����ѽ���ɵ���ϩ����ϩ�ȣ�
������Hg2+�ķ�ˮ�м���Na2S������HgS�ij��������ӷ���ʽΪHg2+ + S2��= HgS����
�ܷϱ�ֽ���������������ǿɻ��������������������ã�����ҩƷ���ɻ��գ����뵹�����������ռ�ֵ����ѡab��
���㣺������ˮú���ķ�Ӧ����֬ˮ�������жϣ����ӷ���ʽ����д�������������ж�


 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��������������Һ���ܴ����������
| A��Fe3+��SCN����I����K+ |
| B��K+��Al3+��SO42����MnO4�� |
| C��H+��NO3����Fe2+��Na+ |
| D��Cu2+��NH4+��Br����OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ij��Һ��ֻ���ܺ���Fe2+��A13+��NH4+��CO32����AlO2����SO32����SO42����C1���е������֣�����ˮ�ĵ��룩������Ũ�Ⱦ�Ϊ0.2mol��L-1����ȡ����Һ����ϡ������ǿ����X��Һ������������������ȡX��Һ��������ʵ�飺
��X��Һ�еμ�Ba(NO3)2��Һ�������������ɫ����A����ɫ����A��A�����������ɫ�����ˣ������ҺA
������ҺA�м��������NaOH��Һ�������塢����B�����˻����ҺB
������ҺB��ͨ������CO2�����г���C����
������˵������ȷ���ǣ� ��
A����ȷ������C�ijɷ�
B����ȷ��ԭ��Һ���Ƿ���Cl����A13+
C����ҺA�д��ڵ���������Ba2+��Fe2+��NH4����H��
D��ԭ��Һ�д���Fe2+��NH4����Cl����SO42��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣���֪��Ӧ��3NO2+H2O==2HNO3+NO���ش��������⣺
��1���÷�Ӧ�����ӷ���ʽΪ ��
��2���������뻹ԭ����������Ϊ �����������뻹ԭ��������ʵ���֮��Ϊ ��
��3���ڱ�״���£�3.36L NO2��H2O��ȫ��Ӧת�Ƶĵ�����ĿΪ ��
��4��д��HNO3��ʯ��ˮ��Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣������������ܶ࣬����H2SO4��H2SO3��SO2��Na2SO3��BaSO4��CuSO4��Na2SO4��7�ֳ����ĺ�����ش��������⣺
��1��д����Ԫ�������ڱ���λ��______________
��2��Ba2+�о綾��ij��������һ�𡰶��ձ����¼����������ձ�̯���������ձ��Ĺ�������̼�ᱵ�����ɷ�ʹ�ã����¶���ʳ���ձ����ж�����д��̼�ᱵ��θ�ᣨ�������ʾ����Ӧ�����ӷ�Ӧ����ʽ���� ������������
��3�������£�����������Ũ�����У����������α��Ͻ���Ϊ�����˶ۻ�����������Ϊδ������Ӧ��Ϊ��֤�˹��̣�ijͬѧ����˼�������������ʵ�飺����Ũ���ᴦ����������ϴ��������CuSO4��Һ�У������������� �����������������������������˶ۻ���
��4������SO2����Ⱦ�����Ϊ�����ҹ�����̽����һ����������CO��ԭSO2�õ�������ķ�������ȥSO2���÷�Ӧ�Ļ�ѧ����ʽ������������ ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ˮ�к���������[Au(CN)2]+����������CN���ж���CN-��H+�������HCNʱ���䶾�Ը�ǿ���ش��������⣺
��1��HCN�ĵ��뷽��ʽΪ______________________NaCN��Һ��pH_____7(�< > =��)
��2����������ʵĵ��뷽��ʽ���ƣ�[Au(CN)2]+Ҳ�������������룬��һ�����뷽��ʽΪ_________
��3)�������ַ�ˮ���ڼ��������£�NaClO��CN������Ϊ̼����͵����������ӷ���ʽΪ��__________
��4)�����������£�ClO��Ҳ������CN--,��ʵ�ʴ�����ˮʱȴ�������������½��е�ԭ����_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ڻ�ҩ��ըʱ�������ֻ�ѧ��Ӧ��������Ҫ��ѧ��Ӧ����ʽΪ��
2KNO3��3C��S K2S��N2����3CO2��
K2S��N2����3CO2��
��1��������Ӧ�У�ԭ�Ӱ뾶��С��Ԫ����ԭ�ӽṹʾ��ͼΪ________________��
��2��������Ӧ�������У����ڷǵ���ʵ���________��
��3��������Ӧ�У�ÿ����1 mol�����������KNO3������Ϊ ������1λС������
��4���ռ��ڻ�ҩ��ը����̳����������ֱ������Ը��������Һ��Ӧ������ʹ��Һ�Ϻ�ɫ��ȥ��
���̳�����ʹ���Ը��������Һ��ɫ�������� ���ѧʽ����
��������������Ը��������Һ��Ӧ�����ӷ���ʽ ��
��������ͼװ�ü�����������е�CO���壬һ��ʱ��۲쵽B�г��ֺ�ɫ��Pd������д��B�з�����Ӧ�Ļ�ѧ����ʽ ��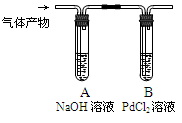
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ֿ���������A��B��C��D��E���������������������ӻ�����ͬ���ֱ�������������K����Fe3����Cu2����Ba2����Al3��������������Cl����OH���� ��
�� ��X�е�һ�֡�
��X�е�һ�֡�
(1)ijͬѧͨ���ȽϷ�������Ϊ�������Ϳ��ж����б��е�����������________��________��
(2)����C�к�������X��Ϊ��ȷ��X���ֽ�(1)�е��������ʼ�ΪA��B����C��A����Һ���ʱ������ɫ��������ó����е�������ϡHNO3�����������ܽ⣬ʣ���ɫ���塣��XΪ________(����ĸ)��
A��Br�� B��
C��CH3COO�� D��
(3)��19.2 g CuͶ��װ������D��Һ���Թ��У�Cu���ܽ⣬�ٵμ�ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֣�д��Cu�ܽ�����ӷ���ʽ��________________����Ҫ��Cu��ȫ�ܽ⣬���ټ���H2SO4�����ʵ�����________��
(4)���ö��Ե缫���C��D�Ļ����Һ�����ʵ����ʵ�����Ϊ0.1 mol����������ϵ�л���ͨ��������������������m��ͨ�����ӵ����ʵ���n�Ĺ�ϵ��(������������ֵ)
(5)E��Һ������ᷴӦʱ��������ɫ���ʣ��÷�Ӧ�����ӷ���ʽΪ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��̽�ն�Ա���������ᣬ��С���߽��˻�ѧ�Թ�����֪�����߳�������Ϊ�Թ������ࡰ���˵�Ұ�ޡ�(���������ᷴӦ�����ʻ�ˮ��Һ)���������ܿ����ǣ��������ͨ����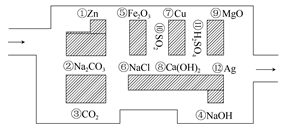
(1)����������߳��Թ� (����ͼ������ǰ���������������ʾ���ߵ�·��)��
(2)���ܡ��Ե�������Ļ�ѧ��Ӧ�У���������кͷ�Ӧ���� ��������������ԭ��Ӧ���� ���������ܡ��Ե������������ ��д���÷�Ӧ�����ӷ���ʽ�� ��
(3)�ڲ��������ᷴӦ�������У����ڵ���ʵ��� �����ڷǵ���ʵ��� (��д�������)��
(4)��������ỻ���������������������߳���·�ߡ��߳�������Թ���Ϊʲô�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com