
����Ŀ�������£��������м��ַ�Ӧ����16H����10Z����2XO![]() ===2X2����5Z2��8H2O����2A2����B2===2A3����2B��2B����Z2===B2��2Z������������Ӧ���ж����н��۴������
===2X2����5Z2��8H2O����2A2����B2===2A3����2B��2B����Z2===B2��2Z������������Ӧ���ж����н��۴������
A. ��Һ�пɷ�����Z2��2A2��===2A3����2Z��
B. ������ǿ����˳��Ϊ��XO![]() >Z2>B2>A3��
>Z2>B2>A3��
C. Z2���٢���Ӧ��Ϊ��ԭ��
D. X2����XO![]() �Ļ�ԭ����
�Ļ�ԭ����
���𰸡�C
��������A������X�Ļ��ϼ۽��ͣ���������XO4-��Z2������BԪ�صĻ��ϼ۽��ͣ���������B2��A3+������ZԪ�صĻ��ϼ۽��ͣ���������Z2��B2����������Z2��A3+����ӦZ2+2A2+=2A3++2Z-�ɷ�������A��ȷ��B������X�Ļ��ϼ۽��ͣ���������XO4-��Z2������BԪ�صĻ��ϼ۽��ͣ���������B2��A3+������ZԪ�صĻ��ϼ۽��ͣ���������Z2��B2����������XO4-��Z2��B2��A3+����B��ȷ��C������ZԪ�صĻ��ϼ����ߣ���Z2Ϊ�����������ZԪ�صĻ��ϼ۽��ͣ���Z2Ϊ����������C����D����Ӧ����XԪ�صĻ��ϼ۽��ͣ���XO4-Ϊ����������X2+��XO4-�Ļ�ԭ�����D��ȷ����ѡC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������գ�
(1)�ڱ�״���£�448 mLij���������Ϊ0.64 g�������������Է�������Ϊ________��
(2)ij�Ȼ������Ȼ��ƵĻ��Һ����֪c(Fe3��)��0.2 mol��L��1��c(Cl��)��1 mol��L��1����c(Na��)Ϊ_____________��
(3)19 gij���۽������Ȼ���RCl2�к���0.4 mol��Cl������R�����ԭ������Ϊ__________��
(4)V L Fe2(SO4)3��Һ�к�Fe3�� m g������Һ��SO42-�����ʵ���Ũ��Ϊ___________mol��L��1��
(5)�����������У�����1 L��5 mol NH4Cl��1.6 mol KCl��2.4 mol K2SO4��ijӪ��Һ������KCl��NH4Cl��(NH4)2SO4���ƣ�����KCl��NH4Cl�����ʵ����ֱ�Ϊ________��________��
(6)��ͼ��ʾ�� �ֱ����ܱ������ڿ��ƶ����������߳������(��֪�������ռ���������ݻ���1/4)��H2��O2�Ļ�����壬�ڱ�״���£�����H2��O2�Ļ�������ȼ���������������ָ�ԭ�¶Ⱥ����һ�ͣ�������������롣��ԭ��H2��O2�����֮�ȿ���Ϊ_________________��
�ֱ����ܱ������ڿ��ƶ����������߳������(��֪�������ռ���������ݻ���1/4)��H2��O2�Ļ�����壬�ڱ�״���£�����H2��O2�Ļ�������ȼ���������������ָ�ԭ�¶Ⱥ����һ�ͣ�������������롣��ԭ��H2��O2�����֮�ȿ���Ϊ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺

��1����Ũ������HCl�����ʵ���Ũ��Ϊ________mol��L��1��
��2��ȡ����������ĸ�����ʱ�������������в�����ȡ����Ķ��ٶ��仯����________��
A����Һ��HCl�����ʵ�����������������B����Һ��Ũ��
C����Һ��Cl������Ŀ�������������� ��D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����500mL���ʵ���Ũ��Ϊ0.400 mol/L��ϡ���ᡣ
�ٸ�ѧ����Ҫ����Ͳ��ȡ________mL����Ũ����������ơ�
��ʵ�鿪ʼʱ����Ҫ���_____________��
�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿(������������ƫ��������ƫС��������Ӱ����)����
a.����Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�档��____��
b.�ܽ⡢ת�ơ�ϴ��ʱ����Һ������������____��
c.����ʱ��500mL����ƿ��ϴ��������������ˮ����____��
d.����ʱ���ӿ̶��ߡ���____��
e. ���ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ����____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѧ����ѡ��5���л���ѧ������
�����㶹����һ����������Ѫ�ܼ�����ҩ����谭Ѫ˨��չ�������㶹�ؿ���ͨ�����·����ϳ�(���ַ�Ӧ����ʡ��)��

��֪��
��1����Ӧ���ķ�Ӧ������__________����Ӧ���ķ�Ӧ����Ϊ____________�����㶹���к��й����ŵ�����Ϊ_____________��
��2����Ӧ���Ļ�ѧ����ʽΪ________________________________
��3����Ӧ���Ļ�ѧ����ʽΪ_________________________
��4������E���ʣ�����˵����ȷ����______ (����ĸ���)��
a.���������Ը��������Һ����D��E
b.�ں˴Ź������������������շ�
c.���Է����ӳɷ�Ӧ���ۺϷ�Ӧ��������Ӧ����ȥ��Ӧ
d.����˳���칹
��5��д��G�Ľṹ��ʽ___________________��
��6�����ӽṹ��ֻ����һ��������ͬʱ��������������G��ͬ���칹�干��_______�֡�
�������Ȼ�����Һ������ɫ��Ӧ��
������̼��������Һ��Ӧ���ɶ�����̼���塣
���У������ϵ�һ�ȴ���ֻ�����ֵ�ͬ���칹��Ľṹ��ʽΪ__________��
��������G�ĸ���ͬ���칹���ѡ�õ�������________(�����)��
a����������� b�������� c��Ԫ�ط����� d���˴Ź�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ƣ�Na2S2O5���dz��õ�ʳƷ��������֮һ��ij�о�С���������ʵ�飺
ʵ��һ ���������Ƶ���ȡ
������ͼװ��(ʵ��ǰ�ѳ���װ���ڵĿ���)��ȡNa2S2O5��װ�â�����Na2S2O5���������������ķ�ӦΪ��Na2SO3��SO2��Na2S2O5

��1��װ��I�в�������Ļ�ѧ����ʽΪ__________________��
��2��Ҫ��װ�â��л���������ľ��壬�ɲ�ȡ�ķ��뷽����_________��
��3��װ�â����ڴ���β������ѡ�õ������װ��(�г���������ȥ)Ϊ________(�����)��

ʵ��� ���������Ƶ�����
Na2S2O5����ˮ������NaHSO3��
��4��֤��NaHSO3��Һ��HSO3���ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________(�����)��
a���ⶨ��Һ��pH b������Ba(OH)2��Һ c����������
d������Ʒ����Һ e������ɫʯ����ֽ���
��5������Na2S2O5�����ڿ������ѱ�������ʵ�鷽����____________��
ʵ���� ���Ѿ��п��������������IJⶨ
��6�����ѾƳ���Na2S2O5�������������ⶨij���Ѿ��п��������IJ�����(������SO2����)�ķ������£�

(��֪���ζ�ʱ��Ӧ�Ļ�ѧ����ʽΪSO2��I2��2H2O��H2SO4��2HI)
�ٰ���������ʵ�飬���ı�I2��Һ25.00 mL���ô�ʵ������Ʒ�п��������IJ�����(������SO2����)Ϊ________________g��L��1��
��������ʵ������У����в���HI���������������ý��____(�ƫ�ߡ���ƫ�͡����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����֪��״����1.505��1023��X������ӵ�����Ϊ8g����X��������Ϊ________________��Ħ������Ϊ__________��
��2����20g�ռ����Ƴ�500mL��Һ�������ʵ���Ũ��Ϊ___________________mol/L������ȡ��1mL�������ʵ���Ũ��Ϊ______________________mol/L��������________________g��������1mL��Һ��ˮϡ�͵�100mL��������Һ�����ʵ����ʵ���Ũ��Ϊ_____________mol/L�����к�Na+__________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л������Է�������Ϊ102��
(1)���ⶨ�����л��ﺬ̼���⡢������Ԫ�أ����к������������Ϊ9.8%����������ԭ�Ӹ���Ϊ��ԭ�ӵ�5�������л���ķ���ʽ��________�������л����������Ѽ������ܷ���������Ӧ������л���Ĺ����ŵ�����________________��д�����л��������Ƶ�������ͭ�ķ�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
(2)��������ײⶨ�����л�������к���һ���Ȼ���һ���ǻ���һ��̼̼˫����������ܵĽṹ��ʽΪ_____________________________________________________(��ʾ���ǻ���������̼̼˫����)��
(3)���ⶨ�����л���ֻ��̼��������Ԫ�أ�������ԭ�Ӿ���ͬһƽ�棬��д�����л���Ľṹ��ʽ��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ�����ʵ���Ũ�ȵ�ϡ���ᣬ���ƫ�ߵ���
A. ������ƿ�ж���ʱ�����ӿ̶���
B. ����Ͳ��ȡŨ����ʱ�����ӿ̶���
C. ת����Һ����δϴ���ձ��Ͳ�������ֱ�Ӷ���
D. ���ݺ������ƿ����ҡ��������Һ����ڿ̶����ּ�ˮ���̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͨ��״���£�CO��һ����ɫ����ζ���ж������壬������ˮ�����ᡢ�����Һ������Ӧ���ƾ���ƿ�����������Դ����ȷ������ͼ��ʾ��װ�ý���ʵ�飬������֤ij�������ijɷ���CO2��CO��ÿ��װ������һ��������ش��������⣺
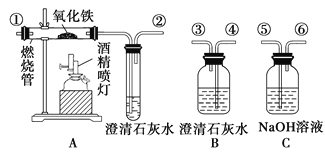
��1������װ�õ��ܿڵ�˳���������__________________��β����������ܽӿڴ�������
��2��֤��ԭ���������CO2���ڵ�ʵ��������__________________________________��
֤��CO���ڵ��йط�Ӧ�Ļ�ѧ����ʽ��__________________________________________��
��3����ͬѧ�������BӦ��ʹ��һ�Σ�����Ϊ�е�����________��������������û�����������������ɣ�
_________________________________________________________________��
��4����ʵ��β�������ķ�����________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com