
| �ζ����� | ���������mL�� | NaOH��Һ���������mL�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.00 | 18.10 |
| 2 | 20.00 | 0.00 | 16.30 |
| 3 | 20.00 | 0.00 | 16.22 |
���� ��1�����ݼ�ʽ�ζ�����װҺǰӦ�ô�װҺ������ϴ������c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��жϣ�
��2�����ݾ�ȷ��ȡҺ�������������õζ��ܣ�����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��жϣ�
��3�������к͵ζ��У��۾�Ӧע�ӵ�����ƿ����Һ��ɫ�仯��������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��4������c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��жϣ�
��5���ȸ������ݵ���Ч�ԣ���ȥ��1�����ݣ�Ȼ�����2��3��ƽ������V��NaOH�������Ÿ���NaOH��HCl�����㣮
��� �⣺��1����ʽ�ζ�����װҺǰӦ�ô�װҺ������ϴ��������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶������ϣ���ʽ�ζ���δ�ñ�NaOH��Һ��ϴ��ֱ��ע���NaOH��Һ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$������c������ƫ��
�ʴ�Ϊ���٣�ƫ��
��2����������ԣ���ȡ20.00mL����ҺӦʹ����ʽ�ζ��ܣ�����ƿװҺǰ��������������ˮ��V���������䣬����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$������c���������䣻
�ʴ�Ϊ����ʽ�ζ��ܣ���Ӱ�죻
��3���к͵ζ��У��۾�Ӧע�ӵ�����ƿ����Һ��ɫ�仯���ζ�ʱ������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣬���Ե��������һ��NaOH��Һ����Һ����ɫ��Ϊ�ۺ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ����ƿ����Һ��ɫ�仯����ƿ����Һ����ɫ��Ϊdz��ɫ������Ӳ���ɫ��
��4�����ϱ����Կ�������1�εζ���¼��NaOH��Һ������Զ��ں����ε��������õ�����Ũ��ƫ��
A���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������ݣ���������������Һ���ƫ����������Ũ��ƫ��A��ȷ��
B����ƿ�ô���Һ��ϴ������Һ�����ʵ���ƫ��������������Һ���ƫ����������Ũ��ƫ��B��ȷ
C����NaOH��Һ����ʱ��������в��ֱ��ʣ�Ũ�Ƚ��ͣ����÷�̪Ϊָʾ�������ղ��ﲻ�䣬û��Ӱ�죬��C����
D���ζ�����ʱ�����Ӽ�������������������Һ���ƫС����������Ũ��ƫС����D����
��ѡ��AB��
��5�����εζ����ĵ����Ϊ��18.10mL��16.30mL��16.22����ȥ��1�����ݣ�Ȼ�����2��3��ƽ������V��NaOH��=16.26mL��
NaOH��HCl
1 1
0.2000mol•L-1��16.26mL c��HCl����20.00mL
��ã�c��HCl��=0.1626 mol•L-1��
�ʴ�Ϊ��0.1626��
���� ���⿼���к͵ζ�ʵ�顢�ζ��ܽṹ��ʹ�á��������Լ�����ȣ��ѶȲ���ע�������к͵ζ���ԭ����ζ��ܵĽṹ�����ȣ�


 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
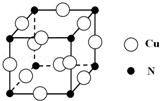 ��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺
��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺ ��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3��
��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3��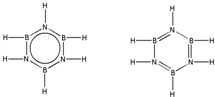 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
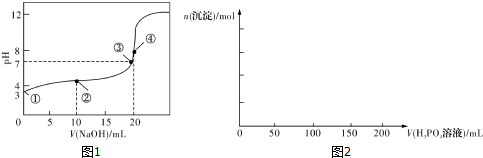
| n��SO32-����n��HSO3-�� | 1��9 | 1��1 | 1��91 |
| pH | 8.2 | 7.2 | 6.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ζ����� | ����Һ�����mL�� | ��NaOH��Һ������¼��mL�� | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 20.00 | 0.40 | 20.40 |
| �ڶ��� | 20.00 | 4.00 | 24.00 |
| ������ | 20.00 | 2.00 | 24.10 |
| ��ѧʽ | AgCl | AgBr | AgI | Ag2S | Ag2CrO4 |
| ��ɫ | ��ɫ | dz��ɫ | ��ɫ | ��ɫ | ��ɫ |
| Ksp | 2.0��10-10 | 5.4��10-13 | 8.3��10-17 | 2.0��10-48 | 2.0��10-12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SiO2��CsCl��CBr4��CF4 | B�� | CF4��CCl4��CBr4��Cl4 | ||
| C�� | ���ʯ������裾�������裾̼���� | D�� | NaF��MgF2��AlF3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
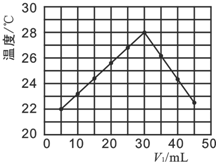 ����к͵ζ�����ѧ��ѧ����Ҫ�Ķ���ʵ��֮һ��ij�о���ѧϰС��ȷ����������ʵ�飬��ȡ1.00g�����Ŀ�������Ʒ���250ml��Һ��ȡ��10.00ml������֪Ũ��Ϊ0.040mol•L-1��������еζ������ʲ������ᷴӦ����
����к͵ζ�����ѧ��ѧ����Ҫ�Ķ���ʵ��֮һ��ij�о���ѧϰС��ȷ����������ʵ�飬��ȡ1.00g�����Ŀ�������Ʒ���250ml��Һ��ȡ��10.00ml������֪Ũ��Ϊ0.040mol•L-1��������еζ������ʲ������ᷴӦ����| ʵ����� | 1 | 2 | 3 | 4 |
| ����������Һ�������mL�� | 20.05 | 20.00 | 22.10 | 19.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ�������£���1 mol N2��3 mol H2��ϣ���ַ�Ӧ��ת�Ƶĵ�����Ϊ6 NA | |
| B�� | 1.5 mol NO2������ˮ��Ӧ��ת�Ƶĵ�����Ϊ1.5 NA | |
| C�� | 6.4 g��S2��S4��S8��ɵĻ���ﺬ��ԭ����Ϊ0.2 NA | |
| D�� | ���³�ѹ�£�11.2 L Cl2����ԭ����ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ܽ�Ͻ�ʱ�ռ���NO�������Ϊ2.24L���ڱ�״���� | |
| B�� | ����Ͻ������������Ϊ9.6g | |
| C�� | ������ȫʱ����NaOH��Һ�����Ϊ150mL | |
| D�� | �μӷ�Ӧ����������ʵ���Ϊ0.4mol |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com