
�л���A��C10H20O2������������ζ��������������ϴ���㲨�ķ��㸳�������֪��
��B������û��֧������D����̼��������Һ��Ӧ�ų�������̼��
��D��E��Ϊ������ͬ�����ŵ�ͬ���칹�塣E���������ϵ���������ԭ��ȡ������һ�ȴ���ֻ��һ�֡���F����ʹ������Ȼ�̼��Һ��ɫ��
��1��D���Է����ķ�Ӧ�� ��ѡ����ţ���
��ȡ����Ӧ ����ȥ��Ӧ�ۼӾ۷�Ӧ ��������Ӧ
��2��C���������Ĺ����ŵĽṹ��ʽ��____��F�����������Ĺ����ŵ�������____��
��3������д��һ����D��E������ͬ����ʽ������������������ʽṹ��ʽ��____
��4��B��C�Ļ�ѧ����ʽ��_ ��C����������Ӧ�����ӷ���ʽΪ ��
��5��E����������������ù�صȡ���֪E���Ʊ�������ͬ���䳣����ͬϵ��ݱ���������2һ����1�������ͼ�����һ����������ȡE���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�____��
��14�֣�ÿ��2�֣�
��1���٢�
��2����CHO��̼̼˫��
��3��
��4��
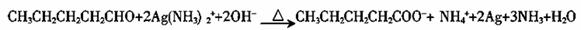
��5��
���������������A��C10H20O2��ΪŨ���������µõ������Բ²�Ϊ�����䲻���Ͷ�Ϊ1����̼��˫����Ϊ���ͽṹ������ת����ϵ�������Ϣ�ó���BΪ����EΪ������̼����ͬ��E�������ⱻ��ȡ��ֻ��һ��һ�ȴ����ṹΪ(CH3)3CCOOH��B����֧����ṹΪCH3CH2CH2CH2CH2OH��B������������������DΪ���B��Ũ���������·�����ȥ��Ӧ����ϩ������ϩ����\
���㣺�������л����ת��Ϊ�����������л���Ľṹ���л������ŵ�ת�������ʡ�ͬ���칹�����д��


 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�д�������ƽ�⣺Br2+H2O HBr+HBrO�л���Ӧ�����Ƚϸ��ӣ����渱��Ӧ��������֪X���ӳɷ�Ӧ�������ɲ���A��D�����������������ա�
HBr+HBrO�л���Ӧ�����Ƚϸ��ӣ����渱��Ӧ��������֪X���ӳɷ�Ӧ�������ɲ���A��D�����������������ա�
��1��A��B�ķ�Ӧ����Ϊ_____________��д��C�Ľṹ��ʽ_________________��
��2����C��ͬ���칹��M�����ܷ���������Ӧ��Ҳ�ܷ���������Ӧ��M��_____��ͬ���칹�壬д�����з��ӽṹ����֧���ķ��ӵĽṹ��ʽ___________________,
��3��д�������л���Ľṹ��ʽ�� F_________________��I______________��
��4�����G��H��ѧ����ʽ��
__________________________________________��
��5�����X��D��ѧ����ʽ__________________________________________��
��6��X����ˮ�ķ�Ӧ�л�����һ���л��������ṹ��ʽΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A�Ǻϳ���Ȼ�ĵ��壬����ʽΪC5H8��A��һϵ�з�Ӧ����(���ַ�Ӧ����ʡ��)��
�ش��������⣺
��1��A�������� ��B�Ľṹ��ʽΪ ��
��2���ڵķ�Ӧ����ʽΪ ��
��3��A��ͬ���칹��������Ȳ�����칹���� �֡�
��4��A��B��Ӧ����������һ����Ԫ��������Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ɱ���ϩ�����з�Ӧ���Ƶ�F��K���ָ߷��ӻ�������Ƕ��dz��õ����ϡ�
��1��J�����������ŵ�����Ϊ ��K�����������ŵĽṹ��ʽΪ
��2���ۺ���F�Ľṹ��ʽ�� ��I�ķ���ʽ�� ��
��3��Eת��ΪG�Ļ�ѧ����ʽ�� ����Ӧ��������
��4����һ�������£�������J����ȥ������ˮ�γ�һ����Ԫ��״�����д���û�����Ľṹ��ʽ ��
��5��д��J ��һ�ַ�������������ͬ���칹��X���ʽ ��
��1mol X������3mol NaOH��Ӧ ��X��������ԭ�Ӻ˴Ź���������4���壬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ȼ��2.8gij�л���A������8.8g CO2��3.6g H2O�������л�������������ܶ�����ͬ������N2��2����
��1������л���ķ���ʽ��
��2�����л�����״ͬ���칹��Ľṹ��ʽΪ�� ��
��3�����ں˴Ź���������ֻ��һ���źŷ壨��ֻ��һ����ԭ�ӣ������ü���ʽ��ʾ�Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������Ļ�����A��C��H��O��Br����Ԫ����ɣ����ӽṹģ������ͼ��ʾ��ͼ��������֮�����߱�ʾ������˫�������й�A�ı仯��ϵ����ͼ������C��ʹFeCl3��Һ����ɫ��H��I��K���ܷ���������Ӧ����ش��������⣺

(1)A�к��й����ŵ������� ��A�ĺ˴Ź��������� �����շ壬1 mol A������ mol NaOH��Ӧ��
(2)����C ��˵����ȷ���� �����ţ���
A�������£�������ˮ�����ӳɷ�Ӧ
B�����������Ը��������Һ��Ӧ
C��һ���������ܷ�����ȥ��Ӧ
D��������һ��ȡ�����ﹲ��3��
(3)д����Ӧ�ܵ����ͣ� ��F�Ľṹ��ʽ�� ��
(4)��д��G+I��K�ķ�Ӧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������M��ij�ֽ�����ʹҩ����AΪԭ�ϵĹ�ҵ�ϳ�·������ͼ��ʾ��
|
 ��֪��
��֪��  +
+

 +CH3COOH
+CH3COOH
 ����һ����ɣ�����Ϳ��ܵ�ԭ��
����һ����ɣ�����Ϳ��ܵ�ԭ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
G��һ�ֹ�ҵ���ܼ�����ϳɵ��������£�
��֪����1��A�����������ֲ�ͬ�����µ���ԭ�ӣ���2��B�Ƿ�����
��1��A�к��������ŵ����� ��F�ķ���ʽ ��
C��D�ķ�Ӧ���� ��B�Ľṹ��ʽ ��
��2��A1����A������ͬ�����ŵ�ͬ���칹�壬ͬ������Ҳ���Ƶ�B��д��A1����B�Ļ�ѧ����ʽ ��
��3��д��D��E�Ļ�ѧ����ʽ ��
��4��F���ʵ���ȥ����H��һ��ҩ���м��壬����H������������ȷ���� ��
a���ܷ���ȡ������ȥ���������Ӿ۷�Ӧ
b����H������ˮ�У���õ���ɫ��Һ
c�������ʵ���H��F��Ӧ����������ܵķ���ʽC18H16O4
d��1mol H��һ����������H2��Ӧ���������H2 4mol
��5��E�ж���ͬ���칹�壬�������������ķ�����ͬ���칹�干�� �֡�
�ټ�����ϡ����������NaOH��Һ��Ӧ ���ܷ���������Ӧ
�۷�����ֻ��һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��10�֣�һ�ֺ��ᾧˮ����ɫ����ɱ�ʾΪB��nH2O�����нᾧˮ����������Ϊ28��57�����þ������ˮ��B��̼���⡢�������������ֱ�Ϊ26��67����2��23����71��1����
��1������ˮ��B�����ʽ��
��2��B��NaOH��Һ�����кͷ�Ӧ������һ����ʽ�κ�һ�����Ρ�0��15 g��ˮ��Bǡ����0��1 mol��L-1NaOH��Һ33��4 mL��ȫ��Ӧ�������Σ���û�����ķ���ʽ����д����ṹ��ʽ��
��3����B��nH2O�е�nֵ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com