
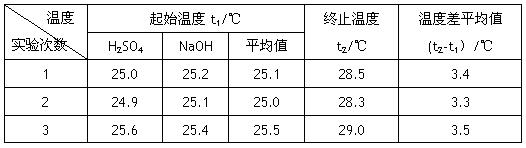


 53������ϵ�д�
53������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
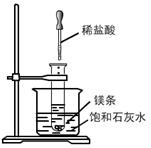
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ȶ��ԣ�Na2CO3 >NaHCO3 | B���ܽ�ȣ�Na2CO3<NaHCO3 |
| C�������ᷴӦ�����ʣ�Na2CO3 <NaHCO3 | D����Է�������Na2CO3 >NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����֪2H2(g)+O2(g) ==2H2O(l)����H=��571.6kJ��mol-1����H2��ȼ����Ϊ285.8kJ��mol-1 |
| B����֪4P�����ף�s��="=" P4�����ף�s������H>0������ױȺ����ȶ� |
| C����20.0g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�28.7kJ����������ϡ�����ϡNaOH��Һ��Ӧ���Ȼ�ѧ����ʽΪ�� NaOH(aq)+CH3COOH(aq) ="=" CH3COONa(aq) + H2O(l) ��H =��57.4kJ��mol-1 |
| D����֪2C(s)+2O2(g)=2CO2(g) ��H1��2C(s)+O2(g)="2CO(g)" ��H2�����H1>��H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2SO3��g�� ��H="-197" kJ��mol-1����ͬһ�¶Ⱥ�ѹǿ�£���ij�ܱ�������ͨ��2 mol SO4��1 mol O2�ﵽƽ��ʱ����Ӧ�ų�������ΪQ1������һ��ͬ���ܱ�������ͨ��1 mol SO2��0.5 mol O2���ﵽƽ��ʱ�ų�������ΪQ2�����й�ϵ��ȷ���ǣ� ��
2SO3��g�� ��H="-197" kJ��mol-1����ͬһ�¶Ⱥ�ѹǿ�£���ij�ܱ�������ͨ��2 mol SO4��1 mol O2�ﵽƽ��ʱ����Ӧ�ų�������ΪQ1������һ��ͬ���ܱ�������ͨ��1 mol SO2��0.5 mol O2���ﵽƽ��ʱ�ų�������ΪQ2�����й�ϵ��ȷ���ǣ� ��A��Q2=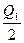 | B��Q2��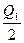 ="197" kJ ="197" kJ | C��Q2��Q1��197 kJ | D��Q1=Q2="197" kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ϡ������0.1mol/LNaOH��Һ��Ӧ��H��(aq)��OH��(aq)=H2O (l) ����H=-57.3KJ/mol |
| B������������Һ�м������������Һ��Fe2+��H2O2��2H+= Fe3+��2 H2O |
| C����Ba(OH)2��Һ���ϼ��뵽KAl(SO4)2��Һ�У���Ӧ���������ʵ������ 3Ba2+��6OH����2Al3+��3SO42��=3BaSO4����2 Al(OH)3�� |
| D���Ȼ�����Һ�м������Ũ��ˮ��Al3+��4NH3��H2O = 2AlO2����4NH4����2 H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ˮ�ֽ������������ | B��������� |
| C������������������������� | D��ϡ�����ϡ����������Һ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������������������۷ֱ���ȫȼ�գ�ǰ�߷ų������� |
B���ɡ�C��ʯī��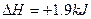 ��mol��1��֪���ʯ��ʯī�ȶ� ��mol��1��֪���ʯ��ʯī�ȶ� |
| C��ϡ��ǿ���ϡ��ǿ����Һ��Ӧ���Ȼ�ѧ����ʽ��Ϊ�� H����OH�� 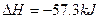 ��mol��1 ��mol��1 |
| D����25�桢101 kPaʱ��1��������ȫȼ������H2O�ų�����142.9kJ���� |
 O2��g����
O2��g����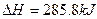 ��
�� mol��1
mol��1�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com