
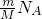 ֪����ͬ���������壬������Ŀ��Ħ�������ɷ��ȣ�������ͬ��H2��NH3��SO2��O3���������У�Ħ�������������庬�еķ��������٣�Ħ���������������Ƕ����������Է��������ٵ��Ƕ�������
֪����ͬ���������壬������Ŀ��Ħ�������ɷ��ȣ�������ͬ��H2��NH3��SO2��O3���������У�Ħ�������������庬�еķ��������٣�Ħ���������������Ƕ����������Է��������ٵ��Ƕ������� ֪����ͬ��������ͬ���������壬���������Ħ�������ɷ��ȣ�������������Ħ��������С��Ħ��������С����������
֪����ͬ��������ͬ���������壬���������Ħ�������ɷ��ȣ�������������Ħ��������С��Ħ��������С���������� =
=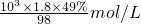 =9mol/L��
=9mol/L��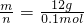 =120g/mol���ʴ�Ϊ��120��
=120g/mol���ʴ�Ϊ��120�� 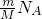 ֪����ͬ���������壬������Ŀ��Ħ�������ɷ��ȣ�����V=
֪����ͬ���������壬������Ŀ��Ħ�������ɷ��ȣ�����V= ֪����ͬ��������ͬ���������壬���������Ħ�������ɷ��ȣ�
֪����ͬ��������ͬ���������壬���������Ħ�������ɷ��ȣ� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�







�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л��ϳ����ִ�����ũҵ������ռ���൱��Ҫ�ĵ�λ���л���F��һ�ָ߷��ӻ�����������ܼ���H�dz��ݼ����м������ǵĺϳ�·�����£�
��֪��
�� R1CH=CHR2  R1COOH + R2COOH ��R1��R2����������
R1COOH + R2COOH ��R1��R2����������
 |
��
��C�ܷ���������Ӧ���ҷ�������֧����
��ش�
��1��E�������������ֻ�ѧ������ͬ��Hԭ�ӣ�ԭ�Ӹ�������������������
��2��D���������������ŵ������ǣ���������������������������������������
��3��д����һ�Ȼ����鵽A�Ļ�ѧ����ʽ��������������������������������������������
��4��д����������������D��һ��ͬ���칹��Ľṹ��ʽ ��
����D������ͬ�Ĺ����ţ��ڷ����о�����������̼ԭ��(�����ĸ���ͬԭ�ӻ���ŵ�̼ԭ�ӣ���Ϊ����̼ԭ��)��
��5��G��H����Է����������63��H����NaHCO3��Һ��Ӧ����0.1moLH������NaOH��Һ��Ӧ������NaOH����������������moL��
��6��B��E��һ������������F�ķ�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�߶���ѧ���Ի�ѧ�Ծ����������� ���ͣ������
�л��ϳ����ִ�����ũҵ������ռ���൱��Ҫ�ĵ�λ���л���F��һ�ָ߷��ӻ�����������ܼ���H�dz��ݼ����м������ǵĺϳ�·�����£�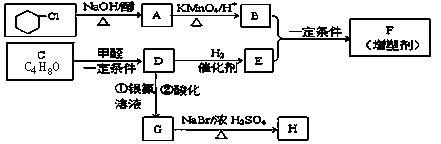
��֪���� ��R1��R2����������
��R1��R2����������
|
 ��C�ܷ���������Ӧ���ҷ�������֧����
��C�ܷ���������Ӧ���ҷ�������֧���� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ���Ի�ѧ�Ծ��������棩 ���ͣ������
�л��ϳ����ִ�����ũҵ������ռ���൱��Ҫ�ĵ�λ���л���F��һ�ָ߷��ӻ�����������ܼ���H�dz��ݼ����м������ǵĺϳ�·�����£�

��֪�� �� ��R1��R2����������
��R1��R2����������
|

��C�ܷ���������Ӧ���ҷ�������֧����
��ش�
��1��E�������������ֻ�ѧ������ͬ��Hԭ�ӣ�ԭ�Ӹ�������������������
��2��D���������������ŵ������ǣ�������������������������
��3��д����һ�Ȼ����鵽A�Ļ�ѧ����ʽ��������������������������������������������
��4��д����������������D��һ��ͬ���칹��Ľṹ��ʽ ��
����D������ͬ�Ĺ����ţ�
�ڷ����о�����������̼ԭ��(�����ĸ���ͬԭ�ӻ���ŵ�̼ԭ�ӣ���Ϊ����̼ԭ��)��
��5��G��H����Է����������63��H����NaHCO3��Һ��Ӧ����0.1moLH������NaOH��Һ��Ӧ������NaOH
���� ������������moL��
��6��B��E��һ������������F�ķ�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�ڵ�һ���ۺ���ϰ�����ۺ��Ծ�����ѧ���֣� ���ͣ������
�л��ϳ����ִ�����ũҵ������ռ���൱��Ҫ�ĵ�λ���л���F��һ�ָ߷��ӻ�����������ܼ���H�dz��ݼ����м������ǵĺϳ�·�����£�

��֪��
�� R1CH=CHR2  R1COOH + R2COOH
��R1��R2����������
R1COOH + R2COOH
��R1��R2����������
 |
��
 |
��C�ܷ���������Ӧ���ҷ�������֧����
��ش�
��1��E�������������ֻ�ѧ������ͬ��Hԭ�ӣ�ԭ�Ӹ�������������������
��2��D���������������ŵ������ǣ���������������������������������������
��3��д����һ�Ȼ����鵽A�Ļ�ѧ����ʽ��������������������������������������������
��4��д����������������D��һ��ͬ���칹��Ľṹ��ʽ ��
����D������ͬ�Ĺ����ţ��ڷ����о�����������̼ԭ��(�����ĸ���ͬԭ�ӻ���ŵ�̼ԭ�ӣ���Ϊ����̼ԭ��)��
��5��G��H����Է����������63��H����NaHCO3��Һ��Ӧ����0.1moLH������NaOH��Һ��Ӧ������NaOH����������������moL��
��6��B��E��һ������������F�ķ�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com