
| 2 |
| 3 |
| n |
| V |
| 2 |
| 3 |
| 1 |
| 3 |
| 2 |
| 3 |


 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ѹ | B����ѹ |
| C������ | D��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijʵ��С���ȡ���²���ⶨij������Ʒ�Ĵ��ȣ�
ijʵ��С���ȡ���²���ⶨij������Ʒ�Ĵ��ȣ�2 3 |
2- 6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
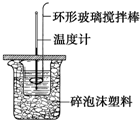 ������ͼ��ʾװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������ͼ��ʾװ�òⶨ�к��ȵ�ʵ�鲽�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Cu��OH��2����Һ�еμ�Na2S��Һ����ɫ�������ɫCu(OH)2(s)+S2-?CuS��s��+2OH- |
| B����H2O2��Һ�У��μ�FeC13��Һ�������ݣ�2H2O2+2C1-=2H2O+O2��+C12�� |
| C������Ӵ���ͭƬ��пƬ����ϡ�����У�ͭƬ���������ݲ�����Cu+2H+=Cu2++H2�� |
| D����CH3COONa��Һ�У��μӷ�̪��죺CH3COO-+H2O=CH3COOH+OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.1mol/L�İ�ˮ��ʹ��̪��Һ��� |
| B��0.1mol/L���Ȼ����Һ��pHԼΪ5 |
| C������ͬ�����£���ˮ��Һ�ĵ����Ա�ǿ����Һ�� |
| D����������ֽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����ʵ���Ũ����ȵ�CuSO4��Һ��NaCl��Һ�������Ϻ���ʯī�缫���е�⣬�������У���Һ��pH��ʱ��t�仯��������ͼ��ʾ��������˵���в���ȷ���ǣ�������
�����ʵ���Ũ����ȵ�CuSO4��Һ��NaCl��Һ�������Ϻ���ʯī�缫���е�⣬�������У���Һ��pH��ʱ��t�仯��������ͼ��ʾ��������˵���в���ȷ���ǣ�������| A��A��pHС��7����ΪCu2+ˮ��ʹ��Һ������ |
| B��BC������������Cl2 |
| C�����������������Ȳ���Cl2�������O2 |
| D��CD�ε���������ˮ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com