
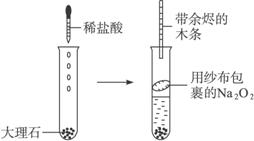
������������:�����Թ��з��뼸�����ʯ,����2��3 mLϡ����
����ȼ�ŵĻ�����CO2������
�������ӽ���ɴ�����õ�Na2O2�����Թ��в�
���ô������ľ������O2�������������������:
(1)Ϊʲô�ڰ���Na2O2��ɴ������֮ǰ�����û����飿
(2)ʵ��ܵ�����������
(3)���ʵ��ڢܲ�δ�۲쵽Ӧ����������ܵ�ԭ����ʲô��
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ijУ��ѧ�о���ѧϰС��Ϊ��֤��ͭ��ϡ���ᷴӦ����һ������������ͼ��ʾװ�ý���ʵ�飬������װ�úͼг�װ�þ�����ȥ���������Ѽ��飬F�����ڹ��������˫��������
ijУ��ѧ�о���ѧϰС��Ϊ��֤��ͭ��ϡ���ᷴӦ����һ������������ͼ��ʾװ�ý���ʵ�飬������װ�úͼг�װ�þ�����ȥ���������Ѽ��飬F�����ڹ��������˫��������| ���� | ���� |
| ��Bװ�����ƣ�ʹ̼�����ϡ����Ӵ� | �������� |
| ��C��������ɫ����ʱ�����̽�Bװ������ | |
| ��A��ͭ˿����ϡ�����У���װ��A���� | װ��A�в�����ɫ���� װ��E�п�ʼʱ����dz����ɫ���� |
| ������F��E�й������ | ��ƿE��������ɫ���� |
| ����һ��ʱ��� | C�а�ɫ�����ܽ� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1����������װ�õ���ȷ˳���ǣ�����ӿڵ���ĸ����I��________��________��________��________��________��________��________��F��________��
��2��ʹ�âۺ�װ�õ������ǣ��ܺ�װ�õ�������________________________________��
��3�������װ�����ռ�������ķ�����________________��������________________��
��4������Ӧǰ����ֱ�����ܵ�����Ϊ100 g��ֱ�������������Ʒ������Ϊ110 g����Ӧ�����ֱ�����ܺ���������Ϊ112.8 g������Ʒ��Na2O2����������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��13�֣�Ϊ��֤��ͭ��ϡ���ᷴӦ��������NO��ijУѧ��ʵ��С�������һ��ʵ�飬��װ������ͼ��ʾ������װ�ú̶�װ�þ�����ȥ����BΪһ���ý���˿�̶��ĸ���ܣ���װ��״̼��ƹ��壻EΪһ���յ�������ƿ��F�����ڹ�������Ĵ�����
��1��ʵ��ʱ����һ��ʵ�����Ϊ����װ��CaCO3�����������ϡ���ᷴӦ����CO2���ò�������Ϊ ����Ӧ�Ļ�ѧ����ʽΪ ��
��װ�õ���ƿ���ȷ��E�п����ѱ��Ͼ���ʵ��������
��
��2���ڶ�������������B, ��A��ͭ˿����ϡ�����У���װ��A���ȣ���װ��A�в�����ɫ���壬�䷴Ӧ�����ӷ���ʽΪ ��������ΪE���ռ����Ŀ�����H2������NO�����֤����
��
��3��ʵ������У�������δ�������������£�E�оͲ�������ɫ��������Ϊ����װ��E������Ʋ�����ɵġ�����Ϊװ��Ӧ�Ľ��ĵط��� ��
��4��װ��D�������� ��
��5����һ����ͭ������ϡ�����ַ�Ӧ�����Եõ�4.48L NO���壨��������ڱ�״���²ⶨ��������Ӧ����Һ���Ϊ100mL����������Һ��Cu2+�����ʵ���Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ�����а��أ��У���һ��ѧ������������ѧ�Ծ� ���ͣ�ʵ����
��13�֣�Ϊ��֤��ͭ��ϡ���ᷴӦ��������NO��ijУѧ��ʵ��С�������һ��ʵ�飬��װ������ͼ��ʾ������װ�ú̶�װ�þ�����ȥ����BΪһ���ý���˿�̶��ĸ���ܣ���װ��״̼��ƹ��壻EΪһ���յ�������ƿ��F�����ڹ�������Ĵ�����

��1��ʵ��ʱ����һ��ʵ�����Ϊ����װ��CaCO3�����������ϡ���ᷴӦ����CO2���ò�������Ϊ ����Ӧ�Ļ�ѧ����ʽΪ ��
��װ�õ���ƿ���ȷ��E�п����ѱ��Ͼ���ʵ��������
��
��2���ڶ�������������B, ��A��ͭ˿����ϡ�����У���װ��A���ȣ���װ��A�в�����ɫ���壬�䷴Ӧ�����ӷ���ʽΪ ��������ΪE���ռ����Ŀ�����H2������NO�����֤����
��
��3��ʵ������У�������δ�������������£�E�оͲ�������ɫ��������Ϊ����װ��E������Ʋ�����ɵġ�����Ϊװ��Ӧ�Ľ��ĵط��� ��
��4��װ��D�������� ��
��5����һ����ͭ������ϡ�����ַ�Ӧ�����Եõ�4.48L NO���壨��������ڱ�״���²ⶨ��������Ӧ����Һ���Ϊ100mL����������Һ��Cu2+�����ʵ���Ũ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com