
����Ŀ���Ǽ��㶹����һ�����Ƶ���ʯ��ҩ��ϳ�·������ͼ��ʾ��
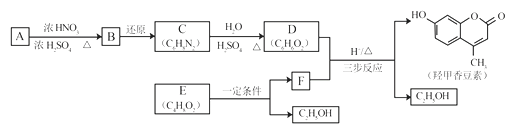
��֪��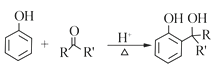
RCOOR'+R'OH![]() RCOOR'+ R'OH��R��R'��R'����������
RCOOR'+ R'OH��R��R'��R'����������
��1��A���ڷ���������ṹ��ʽ��__________________��B�������Ĺ�������_____________��
��2��C��D�ķ�Ӧ������___________________��
��3��E�������ࡣ�����Ҵ�Ϊ�л�ԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�E��д���йػ�ѧ����ʽ��______________________________��
��4����֪��2E![]() F+C2H5OH��F������������
F+C2H5OH��F������������![]() ��___________��
��___________��
��5����D��FΪԭ�Ϻϳ��Ǽ��㶹�ط�Ϊ������Ӧ��д���йػ�����Ľṹ��ʽ��
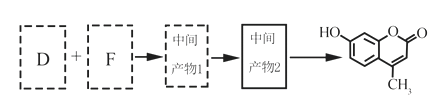 D:______________��F:______________������1:______________������2:______________
D:______________��F:______________������1:______________������2:______________
���𰸡� ![]() ���� ȡ����Ӧ 2CH3CH2OH+O2
���� ȡ����Ӧ 2CH3CH2OH+O2![]() 2CH3CHO+2H2O��2CH3CHO+O2
2CH3CHO+2H2O��2CH3CHO+O2![]() 2CH3COOH��CH3CH2OH��CH3COOH
2CH3COOH��CH3CH2OH��CH3COOH![]() CH3COOCH2CH3��H2O
CH3COOCH2CH3��H2O ![]()
 CH3CH2OOCCH2COCH3
CH3CH2OOCCH2COCH3 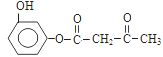
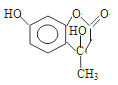
��������(1)A�Ƿ�����������C�Ľṹ��ʽ�Լ�A��B�ķ�Ӧ��������֪A��B�DZ���������Ӧ����֪AΪ�����ṹ��ʽΪ�� ��
��![]() �DZ���������Ӧ����ȡ����Ӧ������������������B�������Ĺ���������������ȷ����
�DZ���������Ӧ����ȡ����Ӧ������������������B�������Ĺ���������������ȷ����![]() �� ������
�� ������
��2��B�е���������ԭΪ��������Ũ������ȵ������£�ˮ�е��ǻ�ȡ�������������ķ�Ӧ��ȡ����Ӧ����ȷ����ȡ����Ӧ��
��3��E�����࣬ԭ�Ͻ�Ϊ�Ҵ�������E�������������ϳɸ�����Ҫ�����Ҵ���������ȩ����ȩ����Ϊ������������Ҵ�����������Ӧ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��2CH3CHO+O2
2CH3CHO+2H2O��2CH3CHO+O2![]() 2CH3COOH��CH3CH2OH��CH3COOH
2CH3COOH��CH3CH2OH��CH3COOH![]() CH3COOCH2CH3��H2O����ȷ����2CH3CH2OH+O2
CH3COOCH2CH3��H2O����ȷ����2CH3CH2OH+O2![]() 2CH3CHO+2H2O��2CH3CHO+O2
2CH3CHO+2H2O��2CH3CHO+O2![]() 2CH3COOH��CH3CH2OH��CH3COOH
2CH3COOH��CH3CH2OH��CH3COOH![]() CH3COOCH2CH3��H2O��
CH3COOCH2CH3��H2O��
��4������ԭ���غ㣬�Ƴ�F�ķ���ʽΪ![]() ��������Ϣ���Լ��Ǽ��㶹�صĽṹ��ʽ���Ƴ�F�Ľṹ��ʽΪ
��������Ϣ���Լ��Ǽ��㶹�صĽṹ��ʽ���Ƴ�F�Ľṹ��ʽΪ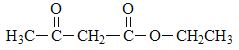 �����˺���
�����˺��� ��������
��������![]() ����ȷ����
����ȷ����![]() ��
��
��5�������Ǽ��㶹�صĽṹ��ʽ���Լ���֪��Ϣ���Ƴ�D�Ľṹ��ʽΪ ����������������F�Ľṹ��ʽΪ
����������������F�Ľṹ��ʽΪ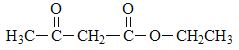 ��F��D�������ơ���֪��Ϣ1���з�Ӧ�����м����1��
��F��D�������ơ���֪��Ϣ1���з�Ӧ�����м����1��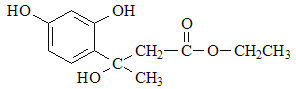 ���м����1�ٷ������ơ���֪��Ϣ2���еķ�Ӧ�����м����2��
���м����1�ٷ������ơ���֪��Ϣ2���еķ�Ӧ�����м����2�� ������м����2�еĴ��ǻ�������ȥ��Ӧ�����Ǽ��㶹�أ���ȷ�𰸣�
������м����2�еĴ��ǻ�������ȥ��Ӧ�����Ǽ��㶹�أ���ȷ�𰸣�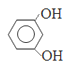 ��CH3CH2OOCCH2COCH3 ��
��CH3CH2OOCCH2COCH3 �� 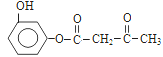 ��
��  ��
��


 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ը�����[��Ҫ�ɷ�ΪFe(CrO2)2��������Al2O3��Fe2O3��SiO2������]Ϊ��Ҫԭ�������ظ����ƾ���(Na2Cr2O7��2H2O��Na2Cr2O7��һ��ǿ������)����Ҫ��������������
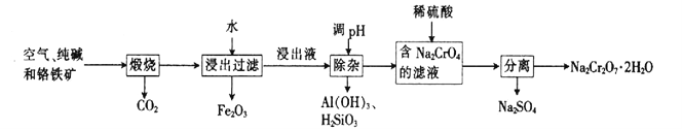
��1���Ǹ�������[Fe(CrO2)2]��Cr�Ļ��ϼ���___________��
��2����������Na2CrO4�Ļ�ѧ����ʽΪ___________��
��3���ữ��ҺNa2CrO4ʱ����ѡ�ø����ԭ����____________________����ƽ��Ƕȷ����ữ��ԭ����______________________��
��4���ù�����ij�ֲ�������������������ʵĻ�ѧʽΪ________________��
��5������ʯī�缫���Na2CrO4��Һ����ʵ��Na2CrO4��Na2Cr2O7��ת������ԭ����ͼ��ʾ��,
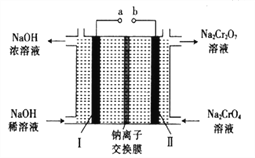
��д�����缫�ĵ缫��Ӧʽ_______________________��
����Na2CrO4ת��Ϊ1malNa2Cr2O7ʱ�����������缫��ת�Ƶ��ӵ����ʵ���Ϊ________��
��3����ȡ2.500g�ظ����ƾ�����������������ˮ���Ƴ�250ml��Һ������ȡ��25.00mL�ڵ���ƿ���������м���10mL2mol��L-1 H2SO4��Һ�������⻯��(���Ļ�ԭ����ΪCr3+)�����ڰ���5min��Ȼ�����100mLˮ������3mL����ָʾ������0.120mol��L-1��Na2S2O3����Һ���еζ�(������Ӧ��I2+2S2O32-=2I-+S4O62-)��
���жϴﵽ�ζ��յ��������______________________��
����ʵ���й���ȥ40.00mL��Na2S2O3����Һ�������ò�Ʒ�Ĵ���Ϊ_____________(�������������������ʲ����練Ӧ)(����3λ��Ч����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H��C��N��O��F��Ca��������Ҫ��Ԫ���������ѧ�����ʽṹ�����ʵ�4����֪ʶ��������:
(1)��̬̼ԭ�ӵĵ����Ų�ʽΪ__,O��F�γɵĻ�������ԭ�Ӽ۲㶼����8���ӽṹ�Ľṹʽ��__,OԪ�صĻ��ϼ�Ϊ________.
(2)Ca��ȼ��ʱ������ɫ���棬����ɫ��Ӧ�����Ĺ�����__(����ա����䡱)���ס�
(3)ijҩ��Ľṹ��ʽΪ![]() ���Ƹ������е�ԭ�ӵ��ӻ��������Ϊ____�������֮��__(��ܡ����ܡ�)�γ������
���Ƹ������е�ԭ�ӵ��ӻ��������Ϊ____�������֮��__(��ܡ����ܡ�)�γ������
(4)CO2����_____(����ԡ��Ǽ��ԡ�)���ӡ�CO32-�Ŀռ乹��Ϊ_____�����以Ϊ�ȵ�����ķ��Ӻ����ӷֱ�Ϊ_________(��дһ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
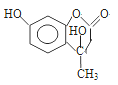
����Ŀ����֪������CΪ��ѧ�����Ļ����
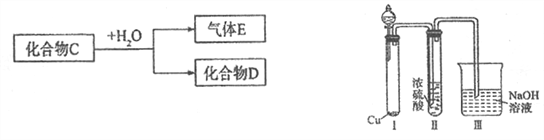
��1����D��һ��ǿ�ᣬ��C��ˮ��Ӧ�Ļ�ѧ����ʽΪ__________________________��
������Ϊ��ŨH2SO4���Ը�������C����ijͬѧΪ����֤�ù۵��Ƿ���ȷ����������ͼװ�ý���ʵ�顣��Һ©����Ӧ����_______________����Һ©������ʵ�飬�����У�ŨH2SO4��δ�����������ݳ����ұ�Ϊ����ɫ������ó��Ľ��ͺͽ�����__________________��
��2����D��һ�ֳ�����ǿ���C�ĵ���ʽΪ:_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����aAn����bB2-�������ӵĺ�����Ӳ�ṹ��ͬ����a����ֵΪ
A.b��n��2B.b��n��2
C.b��n��2D.b��n��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���üױ��ϳ�ij��ʪ����E����������:

��֪:��ϡ����Һ�У������ϵ�±��ԭ�Ӳ�����ȡ��
(1)��������������ȡ����Ӧ�IJ�����_______________��
(2)���������м��˵��Լ�:����________������_______������________��
(3)B�Ľṹ��ʽ��_______________��
(4)�����й�D��������ȷ����_______(����ĸ)
A.�����ֹ����ţ����ǻ����Ȼ�
B.����Cu���������������·���������Ӧ
C.�����γɸ߷��ӻ�����
D.��Ũ��ˮ��Ӧ������ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ��V��W��X��Y��Z��ԭ����������������������Ԫ����ɵľ��������У�n��r��u�����嵥�ʣ������Ϊ�����n�ǻ���ɫ���壬m����ʹʪ��ĺ�ɫʯ����ֽ��������ɫ���壬p����ɫҺ�壬q�ǵ���ɫ���塣���ǵ�ת����ϵ��ͼ��ʾ��
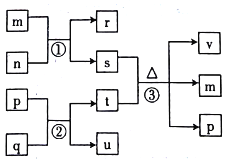
����˵����ȷ����
A. ʵ������ȡm��uʱ�����巢��װ�ÿ�����ͬ
B. ԭ�Ӱ뾶��W>X>Y
C. ��Ӧ�����ھ�Ϊ�û���Ӧ
D. n��t��Һ��Ӧʱ��t����ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
��1����ͼ�ĸ�װ�ó�����ʵ������ȡ���壬������ȡ����ѡ�õ�װ��Ϊ_______________����Ӧ�����ӷ���ʽΪ______________________��
��2������ͼװ����˷ֱ�ͨ���������������ʱ��װ��B�й۲쵽�������Ƿ���ͬ________��������ͬ����������ͬ������װ��A��Ӧ����Һ����ɫ�����������ɫ�����Һ�ж��Ǻ�������ʹ����ɫ______________��д����Ҫ��ʵ�鲽�裩��

��3��װ��F��ʢ�ŵ���ҺΪ______________�������ʵĻ�ѧʽ������װ��E���Լ�Ϊ��˿����ͨ����������˿��ַ�Ӧ�Ļ�ѧ����ʽΪ______________��
��4����ͨ������SO2��ȫ��Ӧ����C�еμӵ�����Һ�����������÷�Ӧ�����ӷ���ʽΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������ɢϵ�ı��������ǣ� ��
A.�����ܷ�����Ӿ����
B.�����ܲ������������
C.��������ֱ����1��100nm֮��
D.������һ���������ܾ۳�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com