
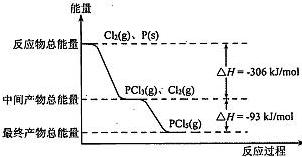
| A�������������䣬�����¶�������PCl5���� |
| B����Ӧ2P��s��+5Cl2��g��===2PCl5��g����Ӧ�ķ�Ӧ�ȡ�H="-798" kJ/mol |
| C��P��Cl2��Ӧ����PCl3���Ȼ�ѧ����ʽΪ2P��s��+3Cl2��g��=2PCl3��g����H="-306" kJ/mol |
| D�������������䣬����PCl5�ֽ����ɣ�PCl3��Cl2�ķ�Ӧ������ѹǿ��PCl5��ת���ʼ�С��ƽ�ⳣ��K��С |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 Ni(CO)4(g)���÷�Ӧ�ġ�H______0 (ѡ�������������)��
Ni(CO)4(g)���÷�Ӧ�ġ�H______0 (ѡ�������������)�� ���¶�(t)�Ĺ�ϵ����ͼ����һ����̼��ԭ����������Ӧ�Ļ�ѧƽ�ⳣ������ʽ�ɱ�ʾΪ��K��______________��800��ʱ���������ױ���ԭ�Ľ�����������___________���÷�Ӧ��ƽ�ⳣ����ֵ(K)����__________��
���¶�(t)�Ĺ�ϵ����ͼ����һ����̼��ԭ����������Ӧ�Ļ�ѧƽ�ⳣ������ʽ�ɱ�ʾΪ��K��______________��800��ʱ���������ױ���ԭ�Ľ�����������___________���÷�Ӧ��ƽ�ⳣ����ֵ(K)����__________��
 CH3OH(g) ��H����90.7 kJ��mol��1
CH3OH(g) ��H����90.7 kJ��mol��1 CH3OCH3(g)��H2O(g) ��H����23.5 kJ��mol��1
CH3OCH3(g)��H2O(g) ��H����23.5 kJ��mol��1 CO2(g)��H2(g) ��H����41.2 kJ��mol��1
CO2(g)��H2(g) ��H����41.2 kJ��mol��1 CH3OCH3(g)��CO2(g)�ġ�H��_______________��
CH3OCH3(g)��CO2(g)�ġ�H��_______________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 O2��g��====H2O��l����H����285kJ��mol��1
O2��g��====H2O��l����H����285kJ��mol��1 ����5O2��g��====3CO2��g����4H2O��l����H����2220.0kJ��mol��1
����5O2��g��====3CO2��g����4H2O��l����H����2220.0kJ��mol��1 ��====H2O��g�� ��H����44.0kJ��mol��1��д������ȼ������CO2����̬ˮ���Ȼ�ѧ����ʽ_________________________________________��
��====H2O��g�� ��H����44.0kJ��mol��1��д������ȼ������CO2����̬ˮ���Ȼ�ѧ����ʽ_________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���ڢ� | C���٢� | D���٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ����H3<0
����H3<0�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
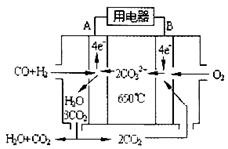
 �ɼ״���CO(g)��2H2(g)
�ɼ״���CO(g)��2H2(g) CH3OH(g)����H
CH3OH(g)����H| 250��: K1=__________ | 300��: K2=0.270 | 350��: K3=0.012 |
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com