

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����10 NA������ת��ʱ���÷�Ӧ�ų�1300kJ������ |
| B����1 NA��ˮ����������ΪҺ��ʱ������1300kJ������ |
| C����2 NA��̼�����õ��Ӷ�����ʱ���ų�1300kJ������ |
| D����8 NA��̼�����õ��Ӷ�����ʱ���ų�1300kJ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��+488.3kJ/mol | B����488.3 kJ/mol |
| C����244.15 kJ/mol | D��+244.15 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��-386 kJ��mol-1 | B��+386 kJ��mol-1 |
| C��-746 kJ��mol-1 | D��+746 kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
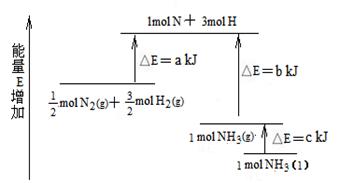
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���缫����ʽ��Cu-2e- = Cu2+�����ܷ�����ԭ����У����ܷ����ڵ����� |
| B��ij�¶��£�10mL 0.1mol/L��H2SO4��Һ��10mL 0.4 mol/L��KOH��Һ��Ϻ�pH=13 |
| C������1mol H-H��N-H��N��N�������յ������ֱ�Ϊa kJ��b kJ��c kJ�� ��2NH3( g) 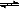 3H2(g)��N2(g) ��H="(3a+c-6b)" kJ/mol 3H2(g)��N2(g) ��H="(3a+c-6b)" kJ/mol |
| D���к͵�����Ĵ�����Һ�����ĵ�pHֵ�İ�ˮ������������Һ������ֱ�ΪV1��V2����V1<V2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��488.3 kJ��mol��1������������ | B����244.15 kJ��mol��1 |
| C��244.15 kJ��mol��1 | D����488.3 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
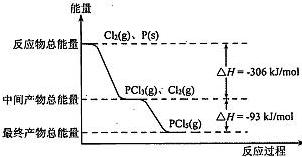
| A�������������䣬�����¶�������PCl5���� |
| B����Ӧ2P��s��+5Cl2��g��===2PCl5��g����Ӧ�ķ�Ӧ�ȡ�H="-798" kJ/mol |
| C��P��Cl2��Ӧ����PCl3���Ȼ�ѧ����ʽΪ2P��s��+3Cl2��g��=2PCl3��g����H="-306" kJ/mol |
| D�������������䣬����PCl5�ֽ����ɣ�PCl3��Cl2�ķ�Ӧ������ѹǿ��PCl5��ת���ʼ�С��ƽ�ⳣ��K��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 4������a mol CH4��CO��H2���������ȫȼ�գ�����CO2�����Һ̬ˮ����CO2��H2O��
4������a mol CH4��CO��H2���������ȫȼ�գ�����CO2�����Һ̬ˮ����CO2��H2O���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com