
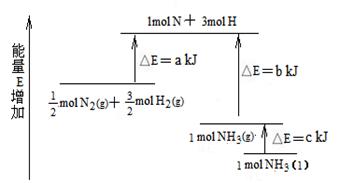
 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ʽ�л�ѧ��������ʾ������ | B����H2>0 |
| C����H2������H1������������������ | D�����á�H1����H2�����ˮ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
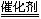 (3��2x)N2��6xH2O
(3��2x)N2��6xH2O (g) ��H��-92.4 kJ��mol-1
(g) ��H��-92.4 kJ��mol-1

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ����
�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��1����֪��
��1����֪�� H1=" 1175.7" kJ��mol-1
H1=" 1175.7" kJ��mol-1 PtF6(g) + e- = PtF6-(g)
PtF6(g) + e- = PtF6-(g)
 H2=" -" 771.1 kJ��mol-1
H2=" -" 771.1 kJ��mol-1 O2+ PtF6-(s) = O2+(g) + PtF6-
O2+ PtF6-(s) = O2+(g) + PtF6-  H3="482.2" kJ��mol-1
H3="482.2" kJ��mol-1  ��ӦO
��ӦO 2��g��+ PtF6 (g) = O2+PtF6- (s)
2��g��+ PtF6 (g) = O2+PtF6- (s)  H="_____________" kJ��mol-1
H="_____________" kJ��mol-1 J���÷�Ӧ���Ȼ�ѧ����ʽΪ________��
J���÷�Ӧ���Ȼ�ѧ����ʽΪ________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2��g��=H2O��1�� ��H=-285.8kJ��mol-1
O2��g��=H2O��1�� ��H=-285.8kJ��mol-1| A��1��1 | B��1��3 | C��1��4 | D��2��3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com