��������������ʽ�������ֵ����ʵ����ı仯����ƽ��ʱ����ֵ����ʵ�����
��1���������ʵ���Ũ�ȶ�����㣻
��2������ת���ʵĶ�����㣻
��3������v=
����v��D����
��4����Ӧ����ﶼΪ���壬���������䣬�����С��ƽ����ϵ�л��������ܶ�����
�÷�Ӧ��Ӧǰ������������䣬�������������䣬ƽ��Ħ���������䣬ƽ����Է����������䣻
��5��Ϊ��Чƽ�⣬Ӧǰ������������䣬����ѧ������ת������ߣ�����n��A����n��B��=1��1���ɣ�
����⣺���ڷ�Ӧ 3A��g��+B��g��

2C��g��+2D��g����
��ʼ��mol����2 2 0 0
�仯��mol����1.2 0.4 0.8 0.8
ƽ�⣨mol����0.8 1.6 0.8 0.8
��1�������������֪��ƽ��ʱB�����ʵ���Ϊ1.6mol������B��ƽ��Ũ��Ϊ
=0.8mol/L���ʴ�Ϊ��0.8mol/L��
��2�������������֪���μӷ�Ӧ��A�����ʵ���Ϊ1.2mol��
��100%=60%���ʴ�Ϊ��60%��
��3��ƽ��ʱD�����ʵ���Ϊ0.8mol��������D��ʾ��ƽ����Ӧ����Ϊv��D��=
=0.2mol/��L?min�����ʴ�Ϊ��0.2mol/��L?min����
��4����Ӧ����ﶼΪ���壬���������䣬�����С��ƽ����ϵ�л��������ܶ�����
�÷�Ӧ��Ӧǰ������������䣬�������������䣬ƽ��Ħ���������䣬ƽ����Է����������䣬
�ʴ�Ϊ�������䣻
��5��Ϊ��Чƽ�⣬Ӧǰ������������䣬����ѧ������ת������ߣ�����n��A����n��B��=1��1���ɣ�
����3A��g��+B��g��

2C��g��+2D��g����֪��C��D��
mol��ת������߿ɵ�A��2mol��B��
mol��
��B�����ʵ���Ϊnmol����2mol����n+
��mol=2mol��2mol�����n=
���ʴ�Ϊ��
��

 2C��g��+2D��g����
2C��g��+2D��g���� 2C��g��+2D��g����֪��C��D��
2C��g��+2D��g����֪��C��D��

 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�
 2C��g��+2D��g������H=Q.2minĩ�ﵽƽ�⣬����0.8mol D��
2C��g��+2D��g������H=Q.2minĩ�ﵽƽ�⣬����0.8mol D��
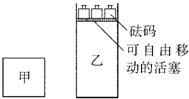 һ���¶��£��п��淴Ӧ��2A��g��+2B��g��?C��g��+3D��g������H��0���ֽ�2molA��2molB�������ΪV�ļ���������2molC��6molD������������ʹ�������ڷ�Ӧ��ʼǰ�����Ϊ2V����ͼ��ʾ�������ַ�Ӧ���������������¶�����ʼʱ��ͬ������˵����ȷ���ǣ�������
һ���¶��£��п��淴Ӧ��2A��g��+2B��g��?C��g��+3D��g������H��0���ֽ�2molA��2molB�������ΪV�ļ���������2molC��6molD������������ʹ�������ڷ�Ӧ��ʼǰ�����Ϊ2V����ͼ��ʾ�������ַ�Ӧ���������������¶�����ʼʱ��ͬ������˵����ȷ���ǣ�������