
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
| Y | ˫ԭ�ӷ��ӵ����ڱ�״�����ܶ�Ϊ1.429g/L |
| Z[ѧ�Ÿ߿���] | ���ʼ��仯�������ɫ��ӦΪ��ɫ |
| W | WԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ������ |
| 1 |
| 2 |
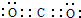 �������д��ڵĦҼ��ͦм��ĸ�������1��1��
�������д��ڵĦҼ��ͦм��ĸ�������1��1��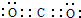 ��1��1��
��1��1�� | 1 |
| 2 |


 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��D��E��X����Ԫ�ص�ԭ���������ε�����A��B��C�Ļ�̬ԭ����L��δ�ɶԵ������ֱ�Ϊ3��2��1��D�Ƕ�������ԭ�Ӱ뾶��������Ԫ�أ�E������Ԫ������Xͬ���ڣ�E��C���γ����ӻ�����侧���ṹ��ͼ��ʾ��Xλ��Ԫ�����ڱ��е������ڢ�B�壮
A��B��C��D��E��X����Ԫ�ص�ԭ���������ε�����A��B��C�Ļ�̬ԭ����L��δ�ɶԵ������ֱ�Ϊ3��2��1��D�Ƕ�������ԭ�Ӱ뾶��������Ԫ�أ�E������Ԫ������Xͬ���ڣ�E��C���γ����ӻ�����侧���ṹ��ͼ��ʾ��Xλ��Ԫ�����ڱ��е������ڢ�B�壮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| X | |||
| Y | Z |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | �������� | �����Լ� | ���뷽�� |
| A | �������л��������Ҵ� | ˮ | ��Һ |
| B | �屽�л��������� | NaOH��Һ | ���� |
| C | Cu��NO3��2��Һ�л�������AgNO3 | ͭ�� | �ᾧ |
| D | K2SO4�����������K2CO3 | ���� | ���� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ij��Һ�еμ�NaOH��Һ�����ȣ��������������ܹ�ʹʪ��ĺ�ɫʯ����ֽ��������ԭ��Һ��һ������NH4+ |
| B����ij��Һ�еμ�AgNO3��Һ����������ɫ��������ԭ��Һ��һ������Cl- |
| C����ij��Һ�еμ�������ˮ���ټ�KSCN��Һ����Һ���ɫ����ԭ��Һ��һ������Fe3+ |
| D����ij��Һ���ȼ��������������ټ��������ữ�����ᱵ��Һ��������ɫ��������ԭ��Һ��һ������SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ȷ����Һ���Ƿ����Cu2+���� |
| B��ԭ��Һ�в���K+��Al3+��CO3I������ |
| C�����ݲ����ֻ��ȷ����Һ��һ������NO3-���� |
| D����������õ��İ�ɫ��������2�ֱ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ɵ�ͭ�����ʵ�����0.2mol |
| B�����ŵ�������Һ��pH��С |
| C��ת�Ƶ��ӵ����ʵ���Ϊ0.4mol |
| D��������Ӧ��40H--4e-=2H20+O2�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com