
��8�֣�
�Ȼ����һ����Ҫ�Ļ���ԭ�ϣ�Ӧ�ù㷺��
��1��ʵ����ͨ����NH4Cl������Ca(OH)2�����Ϲ�����ȡ������
д��ʵ������ȡ�����ķ�Ӧ����ʽ ������
��2����Ũ�Ȼ����Һ��������̨Ļ�����Ż���ԭ���� ������ĸ����
��Ļ�����Ż������ ��Ļ������������ ���Ȼ�立ֽ������������������¶�
���Ȼ�立ֽ��������������˲��ֿ���
| A���٢� | B���ۢ� | C���٢� | D���ڢ� |
 NaCl��N2����2H2Oʵ��װ����ͼ��ʾ���Իش�
NaCl��N2����2H2Oʵ��װ����ͼ��ʾ���Իش�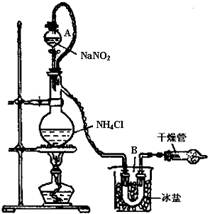

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �¶� | 10�� | 20�� | 30�� | �ܽ�ȣ�20��NaF-4g��0��NH4F-100g�� ����Na2SiF6-����ˮ |
| NH4Cl�ܽ�� | 33.3g | 37.2g | 41.4g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ϻ��мζ���������2010������ڶ���ģ�⿼�Ի�ѧ���� ���ͣ�ʵ����
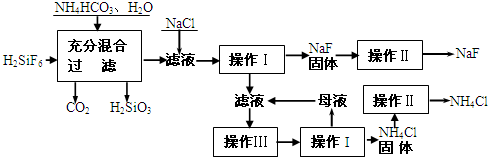
��������һ����Ҫ�ķ��Σ���Ҫ����ũҵɱ������ɱ�����ľ�ķ�������������������ȡ�ʵ���ҿ�ͨ����ͼ��ʾ�������Է����ᣨH2SiF6��������Ϊԭ����ȡ�����ƣ����õ�����Ʒ�Ȼ�泥�

�й�������ˮ���ܽ��(g/100gH2O)���£�
|
�¶� |
10�� |
20�� |
30�� |
�ܽ�ȣ�20��NaF��4 0��NH4F��100�� ����Na2SiF6����ˮ |
|
NH4Cl�ܽ�� |
33.3 |
37.2 |
41.4 |
��ش��������⣺
��1����������Ҫ�õ��IJ��������� ��
��2�����������з���������Ӧ����ѧ����ʽΪ �� ��
��
��3������II�������� �� ��
������ľ�������� ��
��4��������NH4HCO3�����������ԭ���� ������
_______________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ���������ο��Ի�ѧ�Ծ� ���ͣ������
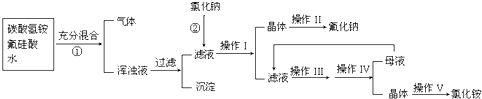
(15��)��������һ����Ҫ�ķ��Σ���Ҫ����ũҵɱ������ɱ�����ľ�ķ�������������������ȡ�ʵ���ҿ�ͨ����ͼ��ʾ�������Է����ᣨH2SiF6��������Ϊԭ����ȡ�����ƣ����õ�����Ʒ�Ȼ�泥�
�й�������ˮ���ܽ��(g/100gH2O)���£�
|
�¶� |
10�� |
20�� |
30�� |
�ܽ�ȣ�20��NaF��4 0��NH4F��100�� ����Na2SiF6����ˮ |
|
NH4Cl�ܽ�� |
33.3 |
37.2 |
41.4 |
��ش��������⣺
��1����������Ҫ�õ��IJ��������� ��
��2�����������з���������Ӧ����ѧ����ʽΪ��
��
��3������II�������� ��
������ľ��������
��4��������NH4HCO3�����������ԭ���� ������
_______________________________________________________________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com