
��17�֣�X��Y��Z��Q��E����Ԫ���У�Xԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�Yԭ�Ӻ����L���������K���������Z�ǵؿ��ں�����������������ߵ�Ԫ�أ�Q�ĺ˵������X��Z�ĺ˵����֮�ͣ�E��Ԫ�����ڱ��ĸ�Ԫ���е縺�������ش��������⣺
��1��X��Y��Ԫ�ط�������Ϊ �� ��
��2��XZ2��YZ2���ӵ��ӻ�����ֱ��� �� ������ṹ�ֱ��� �� ����ͬ������������ˮ�е��ܽ�Ƚϴ���� ��д����ʽ���������� ��
��3��Q��Ԫ�ط����� �������ڵ� ���ڣ����ĺ�������Ų�ʽΪ �����γɻ�����ʱ��������ϼ�Ϊ ��
��4���������ʾʽ д��E���⻯����Һ�д��ڵ��������_______________________��
��1��S C
��2��sp2 sp V�� ֱ���� SO2 ��ΪCO2�ǷǼ��Է��ӣ�SO2��H2O���Ǽ��Է��ӣ����ݡ��������ܡ�ԭ����SO2��H2O�е��ܽ�Ƚϴ�
��3��Cr �� 1s22s22p63s23p63d54s1 +6
��4��F��H��F��H F��H��O O��H��F O��H��O��
��������
��������������������֪��X��S��Y��C��Z��O��Q��Cr��E��F����1��X��Y��Ԫ�ط�������ΪS ��C����2��SO2���ӻ������sp2 ����ṹ��V�Σ� CO2���ӵ��ӻ������sp������ṹ��ֱ���Σ���ͬ����������SO2�Ǽ��Է��ӣ������ܽ��ڼ��Է�����ɵ�����ˮ�У���CO2�����ǷǼ��Է��ӣ����ڼ��Է�����ɵ�����ˮ���ܽ�Ƚ�С��������ΪCO2�ǷǼ��Է��ӣ�SO2��H2O���Ǽ��Է��ӣ����ݡ��������ܡ�ԭ����SO2��H2O�е��ܽ�Ƚϴ�3��Q��Ԫ�ط�����Cr�������ڵ������ڣ����ĺ�������Ų�ʽ��1s22s22p63s23p63d54s1������������Ų�ʽ��֪���γɻ�����ʱ��������ϼ�Ϊ+6�ۡ���4��F���⻯����Һ�д��ڵ����������F��H��F��H F��H��O O��H��F O��H��O��
���㣺����Ԫ�ص��ƶϡ�Ԫ�ط��š�ԭ�ӵĺ�������Ų�ʽ��������ԭ�ӵ��ӻ������ӽṹ������ı�ʾ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ����ʮУ��һ��ѧ�����н�ѧ������⻯ѧ�Ծ��������棩 ���ͣ������
(10��) ������Ҫ��ش����У� ��BaCl2���ڱ�����NH4Cl����Na2SO4���ݸɱ�����Ƭ �������ʡ�
(1) �������ӻ��������__________(����ţ���ͬ)��������ֻ�����Ӽ���������________��
���ڹ��ۻ��������________��
(2) �ۻ�ʱ����Ҫ�ƻ���ѧ������__________���۵���͵���__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʦ���еڶ�ѧ�����п��Ը߶���ѧ�Ծ��������棩 ���ͣ�ѡ����
�ܰѱ������Ȼ�̼����ϩ����ȩ����������������һ���Լ���
A��ˮ B����ˮ C��FeCl3��Һ D��������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʦ���еڶ�ѧ�����п��Ը�һ��ѧ�Ծ��������棩 ���ͣ�ѡ����
����пƬ�ʹ�ͭƬ����ͼ��ʾ��ʽ����ͬŨ�ȵ�ϡ������һ��ʱ��,����������ȷ����
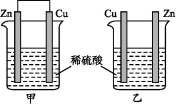
A�����ձ���ͭƬ����������ݲ��� B������ͭƬ������,����ͭƬ�Ǹ���
C�����ձ�����Һ�����Ծ����� D���������ݵ����ʼױ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʦ���еڶ�ѧ�����п��Ը�һ��ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ʵĵݱ��У���ȷ����
A��O��Na��S��ԭ�Ӱ뾶�������� B��LiOH��KOH��CsOH�ļ���������ǿ
C��HF��NH3��SiH4���ȶ���������ǿ D��HCl��HBr��HI�Ļ�ԭ�����μ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ��ʯ���^�߶���ѧ����ͳ���Ծ��������棩 ���ͣ�ѡ����
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��NH4+��Cl����Mg2+��Ba2+��CO32����SO42������ȡ����100mL��Һ��������ʵ�飺
��1����һ�ݼ���AgNO3��Һ�г������ɣ�
��2���ڶ��ݼ�����NaOH��Һ���ռ�������0.05mol��
��3�������ݼ�����BaCl2��Һ�ø������4.3g������������ϴ�ӡ������������Ϊ2.33g��
��������ʵ�飬�����Ʋ���ȷ���ǣ� ��
A��K+һ������ B�������Һ��c(CO32��)Ϊ1 mol/L
C��Cl��һ������ D��Ba2+һ�������ڣ�Mg2+���ܴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ��ʯ���^�߶���ѧ����ͳ���Ծ��������棩 ���ͣ�ѡ����
������Һ������Ũ�ȹ�ϵ�ı�ʾ��ȷ���ǣ� ��
A��NaHCO3��Һ�У�c(H��)��c(Na��)��c(OH��)��c(CO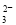 )��c(HCO
)��c(HCO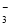 )
)
B��pH��3��CH3COOH��Һ��pH��11��NaOH��Һ�������Ϻ����Һ�У�c(OH��)>c(H��)��c(CH3COO��)
C��0.1 mol��L��1��NH4Cl��Һ�У�c(Cl��)>c(H��)>c(NH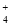 )>c(OH��)
)>c(OH��)
D�����ʵ���Ũ����ȵ�CH3COOH��CH3COONa��Һ�������Ϻ����Һ�У�
2c(Na��)��c(CH3COOH)��c(CH3COO��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ����5��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��1L�ܱ�����������Ӧ��4NH3(g)��5O2(g) 4NO(g)��6HO(g) ��H����Q kJ�� mol��1(Q��0)�������ڲ������ʵ����ʵ���Ũ�����±���
4NO(g)��6HO(g) ��H����Q kJ�� mol��1(Q��0)�������ڲ������ʵ����ʵ���Ũ�����±���
ʱ�䣯Ũ�� | c(NH3)( mol��L-1) | c(O2)( mol��L-1) | c(NO)( mol��L-1) |
��ʼ | 0.8 | 1.6 | 0 |
��2min | 0.6 | a | 0.2 |
��4min | 0.3 | 0.975 | 0.5 |
��6min | 0.3 | 0.975 | 0.5 |
��8min | 0.7 | 1.475 | 0.1 |
��10min | 0.7 | 1.475 | 0.1 |
����˵���������
A����Ӧ�ڵ�2min����4minʱ��O2��ƽ������Ϊ0.1875 mol��L-1��min-1
B����Ӧ�ڵ�2minʱ�ı���ijһ������������������ʹ�ô����������¶�
C����4min����8minʱ�ֱ�ﵽ��ѧƽ�⣬��ƽ�ⳣ����ͬ
D���ڿ�ʼ��Ӧ��ǰ2min�ڣ��÷�Ӧ�ų�0.05QKJ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ�人�����ѧУ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��10�֣�.
��.�±��г���A~R 9��Ԫ�������ڱ��е�λ��(��Ԫ�ط���)��
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 |
|
|
| E |
| F |
|
|
3 | A | C | D |
|
|
| G | R |
4 | B |
|
|
|
|
| H |
|
��1����9��Ԫ���л�ѧ��������õ���__________��
��2��A��B��C����Ԫ�ذ�ԭ�Ӱ뾶�ɴ�С��˳������Ϊ
��3��FԪ���⻯��Ļ�ѧʽ��
��4��GԪ�ظ�BԪ���γɻ�����ĵ���ʽ��
��5��GԪ�غ�HԪ�����ߺ˵����֮����
�����ԭ�ӽṹ���й�֪ʶ��Ԫ�������ɡ�˼�����ش��й�114��Ԫ�صļ������⡣
ԭ�Ӻ�����_____�����Ӳ㣬����������������__________
���ڱ���λ��__________���ڣ�________��
��3�� ����_________Ԫ�أ��������ǽ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com