
���������п�ͼ��ϵ��գ���֪��Ӧ�١������ҹ���ҵ�����е���Ҫ��Ӧ��X������Ϊ��ɫ��ζ��Һ�壻C��ɫ��Ӧ����ʻ�ɫ��JΪ���ɫ������D��E������Ϊ���壬��E��ʹƷ����Һ��ɫ��A�����н�������Ԫ�أ����н���Ԫ�ص���������ԼΪ46.7%��
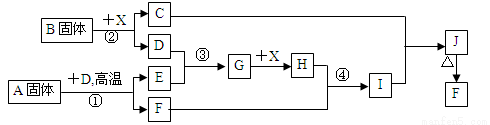
��1��A�Ļ�ѧʽΪ �� F�Ļ�ѧʽΪ ��
��2����Ӧ �۵Ļ�ѧ����ʽ�� ��
��3����Ӧ�ڵ����ӷ���ʽ�� ��
��4����Ӧ�ܵ����ӷ���ʽ�� ��
��5����֪ÿ����16 g E���ų�106.5 kJ��������Ӧ�ٵ��Ȼ�ѧ����ʽΪ��
��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016���㽭ʡ������ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��NAΪ�����ӵ�������ֵ������˵����ȷ����
A�����³�ѹ�£�4.4g��ȩ�����Ҽ���ĿΪ0.7NA
B��2.0gH218O��D2O�Ļ����������������ΪNA
C����״���£�5.6LCO2������Na2O2��Ӧת�Ƶĵ�����Ϊ0.5 NA
D��50ml Ũ��Ϊ12 mol��L-1����������MnO2���ȣ�ת�Ƶĵ�����Ϊ0. 3NA
3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�콭��ʦ���У��Ž�һ�и��������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
ij�ᾧˮ����Ļ�ѧʽΪR��nH2O������Է�������ΪM�� 25 ��ʱ��a g�þ����ܹ�����b gˮ���γ�V mL��Һ�����й�ϵ�в���ȷ���ǣ� ��
A������Һ�����ʵ���������Ϊ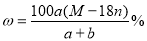
B������Һ�����ʵ���Ũ��Ϊ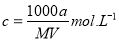
C������Һ���ܶ�Ϊ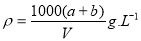
D������Һ���������ܼ���������Ϊ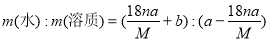
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�찲������ɽ��������ͭ����У�����ϵ�����������ѧ�Ծ��������棩 ���ͣ������
����ѧ��ѡ��3�����ʽṹ�����ʡ�
����ʯ�������鱦�繫��Ϊ�Ĵ�����ʯ֮һ����Ҫ�ɷ�ΪBe3Al2[Si6O18]����������Cr2O3��0.15��0.6%�������γ���ĸ�̡��Իش��������⣺
��1����̬Alԭ���У�������������ܼ���_______����̬Crԭ�ӵļ۵����Ų�ʽ��_____��
��2���á�����������գ�
��һ������ | ���� | �е� | ���Ӱ뾶 |
Be_____B | C��C_____Si��Si | H2S_____H2O | Al3+_____O2- |
��3��BeCl2���ӵĿռ乹����______�����Ķ�����Be2Cl4�ṹ����ͼ��ʾ������Beԭ�ӵ��ӻ���ʽ��_____��
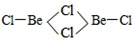
��4��ǿ��ԭ��LiAlH4�ܽ�SiCl4��ԭ��SiH4����д��SiH4�ڿ�������ȼ�Ļ�ѧ����ʽ______��
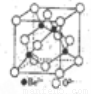
��5��Be������������������ṹ����ͼ��ʾ����֪�����뾧����ܶ�Ϊ��g��cm-3��������Ϊ___cm(��NAΪ�����ӵ�������ֵ���ú�NA���ѵĴ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������������������ѧ��һ��10�»�ѧ���������棩 ���ͣ�ѡ����
.500mL 1mol/L FeCl3��Һ��200mL 1mol/L KCl��Һ�е�Cl-���ʵ���Ũ��֮��
A��5:2 B��3:1 C��15:2 D��1:3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�츣��ʡ������ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
���������ˮ��Һ��Ϊ˫��ˮ����������������ɱ����Ư����ҵ����ԭ�ϵȡ�
ijʵ��С��ȡһ�����Ĺ���������Һ���ⶨH2O2�ĺ�������̽��H2O2��ijЩ���ʡ�Ӧ�á�
�ⶨ���۹���������Һ��H2O2����������
��1����ȡ10.00mL�ܶ�Ϊ�� g/mL�����۹���������Һ��Ӧѡ��������_______������ţ���
A��10mL��Ͳ B����ʽ�ζ��� C����ʽ�ζ��� D��50mL�ձ�
��2����������Һϡ����250mL���������õ��IJ��������ǣ��ձ�����������_________�������ƣ���
��3��ȡ25.00mL��2����ϡ��Һ����ƿ�У�������ϡ�����ữ����c mol/L KMnO4��Һ�ζ���
����ɷ�Ӧ�����ӷ���ʽ��____MnO4-+___H2O2+___H+��___Mn2++___H2O+___��
�ڸ�С��һ���������Ĵβⶨʵ�飬ʵ������¼���£�
ʵ����� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
����KMnO4��Һ���/mL | 25.00 | 25.02 | 20.00 | 24.98 |
�����������ݣ�����ԭ����������Һ��H2O2��������____________________________��
��4�����в����ᵼ�²ⶨ���ƫ�ߵ���_____________��
A���ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ
B����H2O2��Һϡ�ͳ�250��00mL��Һʱ�����ݸ��ӿ̶���
C���յ�ʱ������һ��KMnO4��Һ����Һ�ʺ�ɫ�����ɫ��Һ����һ��H2O2��Һ�Գʺ�ɫ
��.̽��H2O2������
��5�������ⶨԭ����H2O2����__________�ԣ�
��6����Ҫ��֤H2O2���ȶ��ԣ�������____________________________��
��.̽��H2O2��Ӧ��
��7��Ϊ�о���ҵ����������˫��ˮ�����������ڵ�pH����Ӧʱ�������ؼ����������Բ�Ʒ������Ӱ�죬��Ҫ��������ʵ�飬��ʵ�����1�Ǹ����̵�����������������������±�������ʵ�����Ϊ5��6��7������ʵ��������
ʵ����� | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
˫��ˮ��mL�� | 0.5 | 0.4 | 0.6 | 0.5 | |||
pH | 11 | 11 | 11 | 10 | |||
��Ӧʱ�� | 3 | 3 | 3 | 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�����ʡ������ѧ�ڵ������¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
HF������Ӽ����ϣ�ijHF������HF����HF��2����HF��3����϶��ɣ���ƽ����Է�������Ϊ42����HF��3�������������Ϊ�� ��
A��51% B��56% C��57% D��10%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ����ϳ���ʯ�ŵ�һ��ѧ��һ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���������У���ȷ����( )
A��������Ԫ�ص�����һ����������
B����������ԭ��Ӧ�У��ǽ�������һ���ǻ�ԭ��
C��ijԪ�شӻ���̬��Ϊ����̬ʱ����Ԫ��һ������ԭ
D�����������ӱ���ԭ��һ���õ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ����ϳ���ʯ�ŵ�һ��ѧ�߶�����������ѧ���������棩 ���ͣ�ѡ����
ֻ�ı�һ��Ӱ�����أ�ƽ�ⳣ��K�뻯ѧƽ���ƶ��Ĺ�ϵ�����������
A��ƽ���ƶ���Kֵһ���仯 B��Kֵ�仯��ƽ��һ���ƶ�
C��ƽ���ƶ���Kֵ���ܲ��� D��K ֵ���䣬ƽ������ƶ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com