
����ѧ��ѡ��3�����ʽṹ�����ʡ�
����ʯ�������鱦�繫��Ϊ�Ĵ�����ʯ֮һ����Ҫ�ɷ�ΪBe3Al2[Si6O18]����������Cr2O3��0.15��0.6%�������γ���ĸ�̡��Իش��������⣺
��1����̬Alԭ���У�������������ܼ���_______����̬Crԭ�ӵļ۵����Ų�ʽ��_____��
��2���á�����������գ�
��һ������ | ���� | �е� | ���Ӱ뾶 |
Be_____B | C��C_____Si��Si | H2S_____H2O | Al3+_____O2- |
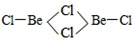
��3��BeCl2���ӵĿռ乹����______�����Ķ�����Be2Cl4�ṹ����ͼ��ʾ������Beԭ�ӵ��ӻ���ʽ��_____��
��4��ǿ��ԭ��LiAlH4�ܽ�SiCl4��ԭ��SiH4����д��SiH4�ڿ�������ȼ�Ļ�ѧ����ʽ______��
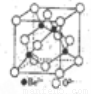
��5��Be������������������ṹ����ͼ��ʾ����֪�����뾧����ܶ�Ϊ��g��cm-3��������Ϊ___cm(��NAΪ�����ӵ�������ֵ���ú�NA���ѵĴ���ʽ��ʾ)��
 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ�߶������У��ģ���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������������뻯ѧʽ�������ȷ����
A�������ơ�NaNO2 B����������-FeO C������-�� D���ƾ�-ȩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�찲��ʡ������ѧ��12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��NA��ʾ�����ӵ�����������˵����ȷ����( )
A���ں���NA ��CH3COO���Ĵ�����Һ�У�H����Ŀ�Դ���NA
B�������£�5.6 L NO��5.6 L O2�Ļ�������к��еķ�����Ϊ0.5NA
C��16.9g BaO2��������������������Ϊ0.3NA
D������1 mol Fe(OH)3���������������к��н�����ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�켪�ֳ��������ѧУ�����ϵ�һ���ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й����ʷ���������ȷ����
��Һ����Һ�ȡ��ɱ����⻯����Ϊ������
�����ȼ������ᡢˮ��������ˮ��Ϊ�����
��������С�մ����ᡢ��ʯ�Ҿ�Ϊ�����
��Na2O2��MgCl2��NaOH��NH4Cl ��Ϊ�����ۼ������ӻ�����
A���ٺ͢� B���ں͢� C���ۺ͢� D���ٺ͢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�츣��ʡ������ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ij��ҵ��ˮ�����±��е�ijЩ���ӣ��Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1mol/L������ֵ����ˮ�ĵ��뼰���ӵ�ˮ�⣩��
������ | K+ Ag+ Mg2+ Cu2+ Al3+ NH4+ |
������ | Cl- CO32- NO3- SO42- I- |
��ͬѧ��̽����ˮ����ɣ�����������ʵ�飺
��ȡ����ɫ��Һ5mL���μ�һ�ΰ�ˮ�г������ɣ��������������ӡ�
���ò�˿պȡ��Һ���ڻ��������գ�����ɫ�ܲ����۲죬����ɫ���档
����ȡ��Һ����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��
������������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ������ƶϲ���ȷ���ǣ�
A���ɢ��жϣ���Һ��һ�������е���������K+��NH4+��Cu2+
B�����м�������������ɫ��������ӷ���ʽ��6I-+2NO3-+8H+�T3I2+2NO��+4H2O
C��ԭ��Һһ��ֻ���е�����I-��NO3-��SO42-��Mg2+��Al3+
D����ȡ100mLԭ��Һ������������NaOH��Һ����ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ��õ��Ĺ�������Ϊ0.4g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʦ����ѧ������ѧ������һ��ģ������ѧ�Ծ��������棩 ���ͣ�ѡ����
����ȷ��ʾ���з�Ӧ�����ӷ���ʽΪ
A������������ϡ�����У�FeS+2H+=Fe2++H2S?
B��NH4HCO3���ڹ�����NaOH��Һ�У�HCO3?+OH?=CO32?+H2O
C������SO2ͨ�뱽������Һ�У�C6H5O?+SO2+H2O=C6H5OH+HSO3?
D������ʯ���ڴ����У�CaCO3+2CH3COOH=Ca2++2CH3COO?+CO2��+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�찲��ʡ������ѧ��12�µ��Ĵ��¿���ѧ�Ծ��������棩 ���ͣ��ƶ���
���������п�ͼ��ϵ��գ���֪��Ӧ�١������ҹ���ҵ�����е���Ҫ��Ӧ��X������Ϊ��ɫ��ζ��Һ�壻C��ɫ��Ӧ����ʻ�ɫ��JΪ���ɫ������D��E������Ϊ���壬��E��ʹƷ����Һ��ɫ��A�����н�������Ԫ�أ����н���Ԫ�ص���������ԼΪ46.7%��
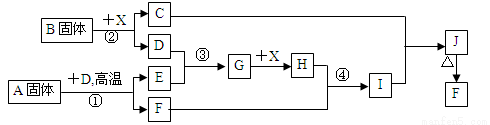
��1��A�Ļ�ѧʽΪ �� F�Ļ�ѧʽΪ ��
��2����Ӧ �۵Ļ�ѧ����ʽ�� ��
��3����Ӧ�ڵ����ӷ���ʽ�� ��
��4����Ӧ�ܵ����ӷ���ʽ�� ��
��5����֪ÿ����16 g E���ų�106.5 kJ��������Ӧ�ٵ��Ȼ�ѧ����ʽΪ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ӱ�ʡ������ѧ�ڵ������¿���ѧ�Ծ��������棩 ���ͣ������
����̼(C0S)����������������Ѭ������
��1���������̼������ĸ�Ԫ���У�ԭ�Ӱ뾶����Ԫ���� (��Ԫ�ط��ţ���
��2��������ʵ�����ڱȽ�C��P����Ԫ�طǽ��������ǿ������ (����ĸ����
A����������ϼ�:P>C
B��ͬ��ͬŨ�ȵ�����Һ������H3P04>H2 C03
C���е�:PH3CH4
(3)����̼ˮ�⼰����Ӧ����������(���ֲ�������ȥ��
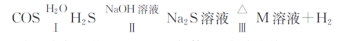
����֪�������£���Ӧ��ÿ����1.7gH2S���壬��Ӧ�ų�����4.76 kj����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
����֪M��Һ����Ԫ�ص���Ҫ������ʽΪS2032-����Ӧ������S2032-�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�켪��ʡ�����ϵ�����ģ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪X��Y��Z��W��Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������W��X�������ǣ� ��
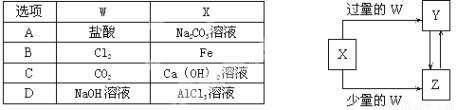
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com