
�ҹ�����ר�Һ�°����ĺ����Ƽ�Ļ�ѧԭ���ǽ�������̼ͨ�백ˮ���Ȼ��Ʊ�����Һ�У��仯ѧ��Ӧ����ʽΪ��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��
��1����ʵ��������������ԭ���ӷ�Ӧ������Һ�з����̼�����ƾ��壬Ӧѡ������װ���е� ��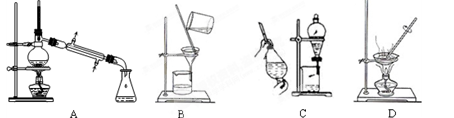
��2��ʵ������̼�����ƾ����У����ܺ��е�����������Cl����NH4+��ʵ���Ҽ���Cl����ѡ�õ��Լ��ǡ���������һ���������ӵķ����� ������ţ���
| A����ˮ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
| B��������������Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
| C��������������Һ�����ȣ������̪�Լ� |
| D��������������Һ�����ȣ�������ɫʯ���Լ� |
��1��B ��2��AgNO3 HNO3 B ��3��2NaHCO3 Na2CO3��CO2����H2O
Na2CO3��CO2����H2O
���������������1��Aװ�������ڷ��뻥�ܵķе㲻ͬ��Һ������װ�ã�Bװ�������ڷ��������ԵĹ���������Ե�Һ�������װ�ã�Cװ��Ϊ��ȡ��Һװ�ã�Dװ��Ϊ�����ᾧװ�á��ʸ��ݺ����Ƽ��ȡ�����ʵ��ص�Ӧ��ѡ�ù���װ�á���ΪBװ�á���2��ʵ�������������������������Ag+��Cl-��Ӧ�����Ȼ�������������Cl-��Ϊ���ų��������ӵĸ���Ҫ��ϡ���ᡣ����ѡ���Լ�ΪAgNO3��HNO3����NH3��ʵ����������Ψһ��ʹʪ��ĺ�ɫʯ����ֽ���������壬�ʼ���NH4+ʱ�ɽ�NH4+ת��ΪNH3��������Һ�м�������������Һ�����ȣ������Թܿ���ʪ��ĺ�ɫʯ����ֽ���顣����ֽ��Ϊ��ɫ����֤�������������к���NH3��ԭ��Һ�к���NH4+����ѡB����3��̼�����Ʋ��ȶ����ȷֽ⡣�ֽ�Ļ�ѧ����ʽΪ��2NaHCO3 Na2CO3��CO2����H2O��
Na2CO3��CO2����H2O��
���㣺��������ķ��뷽������ʵ������Cl����NH4+�ļ����Լ�̼�����ƺ�̼����֮���ת����֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
һ��ⶨ��Ʒ�гɷֺ�����ʵ��Ӧ�ظ�2��3�Ρ�Ϊ�˲ⶨij�������ƹ����л��е�̼
���Ƶ������������ס��ҡ�����λͬѧ�ֱ����������ʵ�鷽����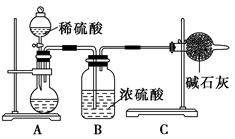
��.��ͬѧ�ķ�����ͼ��ʾ��
(1)��μ���Aװ�õ������ԣ�___________________________________��
(2)��ͬѧ�ظ�����������ʵ�飬�õ�̼���Ƶ��������������ݴ��ڽϴ��ƫ�����Ϊ��������������ƫ�͵�ԭ����________(�����)��
A��װ����ԭ�п����еĶ�����̼����Ҳ����ʯ������
B��װ��������е�ˮ�����Ͷ�����̼����ʯ������
C����Ӧ��ɺ�װ���еĶ�����̼û��ȫ������ʯ������
D������ϡ����������㡢��Ӧ�����
(3)Ϊ���ü�ʵ����������ȷ��������ʵ�鲽�趼��ȷ�������£�����Ϊͼ�е�ʵ��װ��Ӧ����θĽ���___________________________________________��
��.��ͬѧ�ķ����ǣ���ͼ�����ṩ��װ����ѡ��ʵ��װ�ã������ͬѧʵ��װ���е�B��C��ͨ���ⶨ�ų��Ķ�����̼�����(�����Ƕ�����̼����ˮ)�����㡣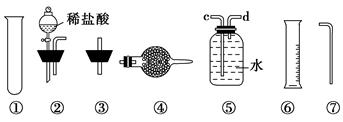
ѡ�����װ�õ�����˳��Ϊ________��
��.��ͬѧ�ķ����ǣ���ȡ��Ʒm g�����ܽ⣬��������Ȼ�����Һ�����ˡ�ϴ�ӡ���ɡ��������ù���n g��
(1)����100 mL 0.10 mol/L BaCl2��Һ��ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ���______(����������)��
(2)�������̼���Ƶ���������Ϊ(��m��n��ʾ)________��
(3)Ca2����Ba2��������ʹCO32��������ȫ���ܷ�ʹ���Ȼ�����Һ�����Ȼ�����Һ��________(��ܡ���)��ԭ���ǣ�______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��Ԫ������Ȼ������Ҫ��������������Ҫ�ɷ�ΪAl2O3��������Fe2O3��FeO��SiO2���С���ҵ�����������Ʊ�����ij�ֻ�����Ĺ����������¡�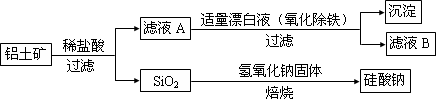
��1������ҺA�м���Ư��Һ��Ŀ��������������������ҺB�����ԡ�
�ټ�����ҺB���Ƿ�����Ԫ�صķ���Ϊ�� ��ע���Լ�������
�ڼ�����ҺA���Ƿ���Fe2�����Լ��� ����ʵ������Ϊ ��
������ҺB�Ʊ��Ȼ��������漰�IJ���Ϊ���ߵμ�Ũ���������Ũ������ȴ�ᾧ�� ����������ƣ���ϴ�ӡ�
��2��SiO2��NaOH�����Ʊ������ƣ��ɲ��õ�װ��Ϊ ������ţ���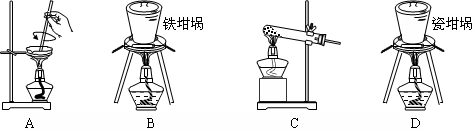
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧ����С��Ϊ�˼���̼���ƺ�̼������������ɫ���壬�ò�ͬ�ķ�����������ʵ�飬��ͼI������ʾ��
��1��ֻ����ͼI������ʾʵ�飬�ܹ��ﵽʵ��Ŀ�ĵ��� ����װ����ţ���
��2��ͼI��ʾʵ��ǰҪ�ȼ��������ԣ�����������Եķ����ǣ��رշ�Һ©�������������ܿڽ���ˮ�У� �������������á�
��3��ͼ����ʾʵ����ܼ������������ʣ����Ƚ�ͼ���ͼ������ʵ�飬ͼ��ʵ����ŵ��� ��
��4������ͼ��ʵ����֤̼���ƺ�̼�����Ƶ��ȶ��ԣ����Թ�B��װ��Ĺ�������� ���ѧʽ����
��5����ȥ̼���ƹ���������̼�����Ƶķ����� ���ѧ����ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij̽��С�������ͼ��ʾװ�ý���Fe����ˮ�����ķ�Ӧ��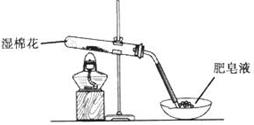
��1��ʵ��ǰ���װ�������Եķ���Ϊ________________________________________________________��
��2������ʵ�������������ʵ�������_____________________________________________��
��3����̽��С���Ϊ���飬����ͼװ�ý��жԱ�ʵ�飬�����þƾ���ơ������þƾ��Ƽ��ȣ���Ӧ�����Ϊ��ɫ��ĩ(������)������ֱ��ò����������ʵ�顣
| ���� | ���� | �������� | �������� |
| 1 | ȡ��ɫ��ĩ����ϡ���� | �ܽ⣬������ | �ܽ⣬������ |
| 2 | ȡ����1����Һ���μ�����KMnO4��Һ | ��ɫ��ȥ | ��ɫ��ȥ |
| 3 | ȡ����1����Һ���μ�KSCN��Һ | ��� | ������ |
| 4 | ����3��Һ�еμ�������ˮ | ��ɫ��ȥ | �ȱ�죬����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�Ȼ��������Ȼ����Ļ�����Ҫ�ⶨ������Ԫ�ص�������������������ʵ�飺
��ͬѧ�����������̽���ʵ�飬�ش��������⣺
��1�����������õ��IJ����������ձ����������⣬�������� �� �����������ƣ�
��2����д��������ˮ���������ӷ�Ӧ����ʽ ��
��3�������������������м��������غ���ȴ�����£�����ʣ���������������������㡣
ʵ���м��������ص�Ŀ���� ��
��4��������������W1g����������Ⱥ������������W2g������Ʒ����Ԫ�ص����������� ��
����ͬѧ����������Բ������·������ⶨ��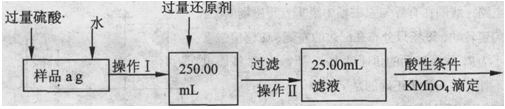
��1���ܽ���Ʒ���������ᣬ�����ӷ���ʽָ������������ԭ���� ��
��2����Ҫ��ʵ��֤��������������Һ�в�����Fe3+������ɿ��Ļ�ѧ������ ��
��3�����ζ��õ�c mol��L KMn04��Һb mL������Ʒ����Ԫ�ص����������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�Ȼ�����Ʒ��������FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�
������������̣��ش��������⣺
��1������I��������Һ�����õ��IJ����������ձ����������⣬�������� �� .�����������ƣ�
��2�����в�������ʹ������ҺŨ��ƫС����________________����д��ţ���
��δϴ���ձ��Ͳ�����
�ڶ���ʱ��������ƿ�Ŀ̶���
������Һǰ����ƿ������������ˮ
��ҡ�Ⱥ���Һ����ڿ̶��ߺ������ˮ����Һ����̶�������
��3����д��������ˮ���������ӷ���ʽ ��
��4����������Ƿ��Ѿ�ϴ�Ӹɾ��IJ����� ��
��5����ԭ��Ʒ����aΪ50g�����Ⱥ����ɫ��������bΪ3g������Ʒ����Ԫ�ص����������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ȵ�������ˮ������Ӧ�����������ɣ�������ͼ��ʾװ�ý������ڸ�������ˮ������Ӧ��ʵ�飬���üķ����ռ����������ɵ���������ش��������⣺
(1)д�����ڸ�������ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
(2)�����C��ʢ�ŵ�ҩƷ�� �� (�ѧʽ)������ܵ� (�m����n��)����g���������ӡ�
(3)��D���������Թ��ռ�������װ��ͼ(����������������ѡ��)��
(4)�����üķ��������ռ���������������������ʵ�������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��1��ʵ�����ö������̸�Ũ���ᷴӦ��ȡ���������ӷ���ʽΪ ��
��2����������dz����������������������£�MnO4������ԭ��Mn2+���ø�����ظ�Ũ���ᷴӦ�������������������ӷ���ʽΪ ��
��3����ʷ�����á��ؿ���������������һ��������CuCl2����������450�����ÿ����е��������Ȼ��ⷴӦ����������Ӧ�Ļ�ѧ����ʽΪ ��
��4������100 mL AlCl3��MgSO4�Ļ����Һ���ֳ����ȷݡ�
�� ������һ���м���10 mL 4 mol/L�İ�ˮ��ǡ����ȫ����������AlCl3�백ˮ��Ӧ�����ӷ���ʽ�� ����������1 mol/L NaOH��Һ��10 mLʱ���������ټ��٣��������ٵ����ӷ���ʽ�� �����ٵij��������ʵ����� ��
�� ����һ���м���a mL 1 mol/LBaCl2��Һ��ʹSO42��������ȫ��a= ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com