




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A������������Һ����һ����AgNO3��Һ���μӰ�ˮ������ǡ���ܽ⡣ |
| B���ڼ���ȩ������Cu(OH)2����Һʱ����һ����CuSO4��Һ�м�������NaOH��Һ |
| C����֤RXΪ����飬��RX���ռ�ˮ��Һ��ϼ��Ƚ���Һ��ȴ���ټ�����������Һ |
| D����ˮ�Ҵ���Ũ���Ṳ�ȵ�170�棬���Ƶõ�����ͨ�����Ը�����أ��ɼ����Ƶõ������Ƿ�Ϊ��ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | ��SO2ͨ����ˮ����ˮ��ɫ����ȹ۲��ܷ�ָ�ԭɫ | ��֤SO2Ư�Ŀ����� |
| B | ����ˮ�������KI��Һ�� | ��֤Cl�ķǽ����Ա�Iǿ |
| C | �������ͭ���õ������Ӳ���Ũ������ | ���ԭ�����֤Fe��Cu���� |
| D | �����Ȼ�狀͵ⵥ�ʵĹ������� | �����ȥ�ⵥ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
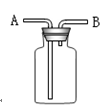
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����������� | ʵ��Ŀ�Ļ���� |
| A | �������ữ��H2O2��Һ����Fe(NO3)2��Һ�У���Һ���ɫ | ��֤��������H2O2��Fe3+ǿ |
| B | ��0.1 mol/L��NaHCO3��Һ�У���2�η�̪��dz��ɫ���ȣ���Һ��ɫ���� | ��֤����ˮ�ⷴӦ�����ȷ�Ӧ |
| C | ��м���ȵ�̼������Һϴ�ӣ���������ˮ��ϴ�ɾ� | ��ȥ��м��������� |
| D | ��������Һ��ͨ������������̼�������� | ���ӵ���������̼�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
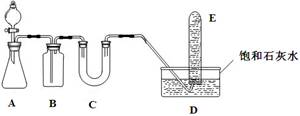
|
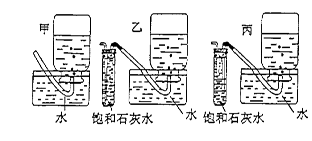
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ˮ����Ũ�����еõ�ϡ���ᣬ��þƬ������̽����Mg�Ļ����� |
| B����NaOH��Һ��������MgSO4��Һ�У��۲�Mg��OH��2���������� |
| C����Mg��OH��2��Һֱ�ӵ�����װ����ֽ��©���й��ˣ�ϴ�Ӳ��ռ����� |
| D����Mg��OH��2����ת��������У�������ϡ���ᣬ�������ɵ���ˮMgC12���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
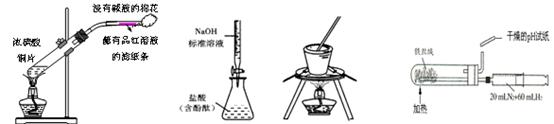
| A����ȡSO2������SO2��Ư���� | B���ⶨ����Ũ�ȼ��� |
| C����ʳ��ˮ����ȡNaCl | D���ϳɰ����������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com