
 4NO��6H2O 2NO��O2===2NO2
4NO��6H2O 2NO��O2===2NO2 2N2��6H2O
2N2��6H2O

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
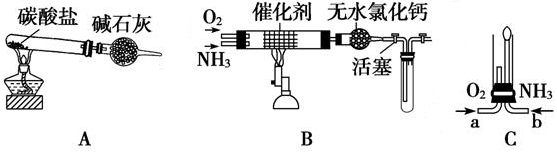
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ݢڢܢۢ� | B���ݢܢڢۢ٢� | C���٢ܢڢݢۢ� | D���٢ڢۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͭ��ϡ���ᷴӦ����ˮ���ռ�NO�� | B��п��ϡ���ᷴӦ������ |
| C��������������Ӧ��NO�������������� | D�������Ȼ����ȡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 R-Br+H2O ��
R-Br+H2O ��| | �Ҵ� | ������ | ������ | 1-�嶡�� |
| �ܶ�/g��cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
| �е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
�鿴�𰸺ͽ���>>
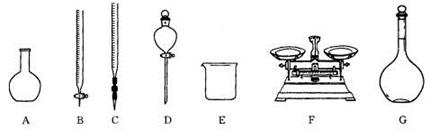
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ���������ϡ��� �����������110�桫140������Ӧ�����ɵõ�S2C12���� S���۵�Ϊ112��8�桢�е�Ϊ444��6�棻S2C12���۵�Ϊ
���������ϡ��� �����������110�桫140������Ӧ�����ɵõ�S2C12���� S���۵�Ϊ112��8�桢�е�Ϊ444��6�棻S2C12���۵�Ϊ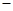 76�桢�е�Ϊ138�档
76�桢�е�Ϊ138�档 2SCl2���� S2C12��ˮ������Ӧ������H2S��SO2��H2SO3��H2SO4�ȡ���ClO3-+5Cl-+6H
2SCl2���� S2C12��ˮ������Ӧ������H2S��SO2��H2SO3��H2SO4�ȡ���ClO3-+5Cl-+6H =3C12��+3H2O ��ش��������⣺
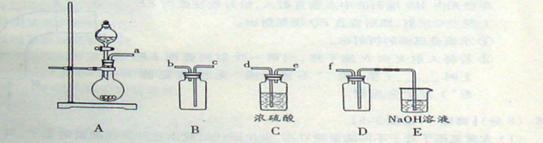
=3C12��+3H2O ��ش��������⣺ ��ʵ��װ����ơ�
��ʵ��װ����ơ�

 ��1��B�������Լ�Ϊ �� C�������Լ�Ϊ ��
��1��B�������Լ�Ϊ �� C�������Լ�Ϊ �� ��2���ڼ���Dʱ�¶Ȳ��˹��ߣ���ԭ���� ��
��2���ڼ���Dʱ�¶Ȳ��˹��ߣ���ԭ���� �� ��3��Gװ�õ������� ��
��3��Gװ�õ������� ���鿴�𰸺ͽ���>>
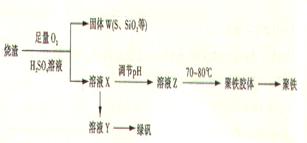
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com