
��10�֣���1���ٸ���ͼʾ��д����Ӧ���Ȼ�ѧ����ʽ�� ��
�ڸ�����ͼ��ʾ������ж�����˵������ȷ���ǣ� ��
A�����Ȼ�ѧ����ʽΪ��CO(g)��H2O(g)===CO2(g)��H2(g)������H����41kJ/mol
B���÷�ӦΪ���ȷ�Ӧ
C���÷�ӦΪ���ȷ�Ӧ
D����H2OΪҺ̬ʱ���䷴Ӧ��ֵС��41 kJ/mol
��2����֪16 g��������ȫȼ��ʱ�ų�148.4 kJ���������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
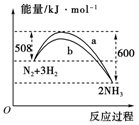
��3����ͼ��ij�¶��£�N2��H2��Ӧ�����������仯������ͼ���÷�Ӧ���Ȼ�ѧ����ʽΪ�� ��
a��b�������߲��������ԭ��ܿ����� ��
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���������Ը��и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��10�֣���1���ٸ���ͼʾ��д����Ӧ���Ȼ�ѧ����ʽ�� ��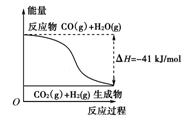
�ڸ�����ͼ��ʾ������ж�����˵������ȷ���ǣ� ��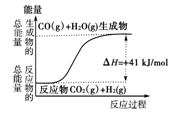
| A�����Ȼ�ѧ����ʽΪ��CO(g)��H2O(g)===CO2(g)��H2(g)������H����41 kJ/mol |
| B���÷�ӦΪ���ȷ�Ӧ |
| C���÷�ӦΪ���ȷ�Ӧ |
| D����H2OΪҺ̬ʱ���䷴Ӧ��ֵС��41 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���������Ը��и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��10�֣���1���ٸ���ͼʾ��д����Ӧ���Ȼ�ѧ����ʽ�� ��

�ڸ�����ͼ��ʾ������ж�����˵������ȷ���ǣ� ��

A�����Ȼ�ѧ����ʽΪ��CO(g)��H2O(g)===CO2(g)��H2(g)������H����41 kJ/mol
B���÷�ӦΪ���ȷ�Ӧ
C���÷�ӦΪ���ȷ�Ӧ
D����H2OΪҺ̬ʱ���䷴Ӧ��ֵС��41 kJ/mol
��2����֪16 g��������ȫȼ��ʱ�ų�148.4 kJ���������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��3����ͼ��ij�¶��£�N2��H2��Ӧ�����������仯������ͼ���÷�Ӧ���Ȼ�ѧ����ʽΪ�� ��

a��b�������߲��������ԭ��ܿ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ס��ҡ����������ֵ����ڵ�ȼ������������������X��Y��Z��W���ֻ����ת����ϵ����ͼ��ʾ����֪��
�ټס��ҡ�����Ϊǰ20��Ԫ�صĵ��ʣ�������Ϊ��̬�������ճ������е�һ�ֳ���������
�ڳ�����XΪ��ɫҺ�壬Y�Ǻ�ɫ���塣
�۱�������ȼ�շ�����ɫ���棬��������ȼ�������ػ�ɫ���̣�W��ˮ��Һ�ʻ�ɫ����ش�
��1������ͼʾ��ϵд������������Ӧ�Ļ�ѧʽ��
�ף��������� ���ң��������� ��
������������ ������������ �� ��
��2��������W����Һ�����ˮ�У���Ӧ�Ļ�ѧ����ʽΪ�������� �������������� ��
��3��д����Y��ȡ���Ļ�ѧ����ʽ�������������� �������������������������� ��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com