
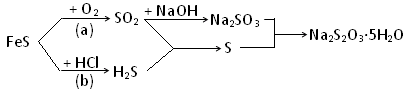
 2Fe2O3+4SO2��Na2SO3+S
2Fe2O3+4SO2��Na2SO3+S Na2S2O3
Na2S2O3| | ��һ�� | �ڶ��� | ������ |
| ��Ʒ������/g | 7��54 | 15��08 | 35��00 |
| ������������/L | 0��672 | 1��344 | 2��688 |
| �������/g | 0��80 | 1��60 | 3��20 |
 2Fe2O3+4SO2, FeS+2HCl=FeCl2+H2S����2H2S +SO2=3S+2H2O��SO2+2NaOH=Na2SO3+ H2O��Na2SO3+S
2Fe2O3+4SO2, FeS+2HCl=FeCl2+H2S����2H2S +SO2=3S+2H2O��SO2+2NaOH=Na2SO3+ H2O��Na2SO3+S Na2S2O3����ǡ����ȫ��Ӧ����Na2SO3��S=1:1�����ݷ���ʽ�������ƣ��ɵõ�FeS�ڷ�Ӧ(a)��(b)�е����۷����Ϊ2:1��
Na2S2O3����ǡ����ȫ��Ӧ����Na2SO3��S=1:1�����ݷ���ʽ�������ƣ��ɵõ�FeS�ڷ�Ӧ(a)��(b)�е����۷����Ϊ2:1�� Na2S2O3��֪Na2SO3��S=1:1�����������Na2SO3�����ʵ���Ϊx������NaOH��Һ����SO2��������Ϊ96%����Ӧ���ĵ�SO2�����ʵ���Ϊx��0��96�������S��ȫ(1- x��0��96)��ȫת��ΪS���ʣ� 1- x��0��96=x�����x=0��4898�����Եõ���Na2S2O3��5H2O����Ϊ��0��4898mol��248g/mol=121��5 g��
Na2S2O3��֪Na2SO3��S=1:1�����������Na2SO3�����ʵ���Ϊx������NaOH��Һ����SO2��������Ϊ96%����Ӧ���ĵ�SO2�����ʵ���Ϊx��0��96�������S��ȫ(1- x��0��96)��ȫת��ΪS���ʣ� 1- x��0��96=x�����x=0��4898�����Եõ���Na2S2O3��5H2O����Ϊ��0��4898mol��248g/mol=121��5 g��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
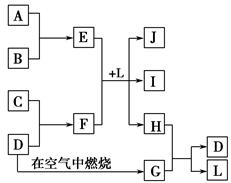
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڢۢܢ� | B���ڢܢݢ� | C���٢ڢܢ� | D���ۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaHCO3��Al(OH)3�������� | B��BaCl2��NaCl |
| C��KClO3��K2SO4 | D��Na2SO3��BaCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
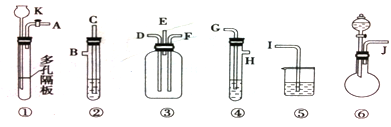
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 Na2S2O3
Na2S2O3
 2NaI+Na2S4O6����Ʒ�е�Na2S2O3��5H2O�Ĵ���Ϊ��������%��
2NaI+Na2S4O6����Ʒ�е�Na2S2O3��5H2O�Ĵ���Ϊ��������%���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������Ǽ���Ũ������ڣ�˵��Ũ���������ˮ�� |
| B��Ũ�������������������˵��Ũ���������ˮ�� |
| C��ͭ��Ũ���Ṳ���д̼�����ζ����ų���˵��Ũ�������ǿ������ |
| D��������Ũ��������������棬˵���������Ũ�����Ӧ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com