
| ʵ�鲽�� | ʵ������ | ��Ӧ�����ӷ���ʽ |
| �������м������NaOH��Һ�� | | �������������� �������������� |
| ���ˣ���������Һ��ͨ�����������̼�� | ������������ ������ | �������������� �������������� |
| �����������ϡ���� | �������������� ���� | |
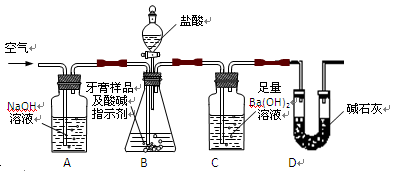
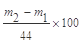 (2��) Dװ�����շ�Ӧ���ɵ�CO2�⣬�������ջӷ�����HCl��ͬʱ�����е�H2O��CO2Ҳ�����Dװ�á�ʹm2-m1ƫ��(2��)��
(2��) Dװ�����շ�Ӧ���ɵ�CO2�⣬�������ջӷ�����HCl��ͬʱ�����е�H2O��CO2Ҳ�����Dװ�á�ʹm2-m1ƫ��(2��)��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
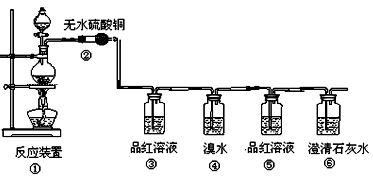
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
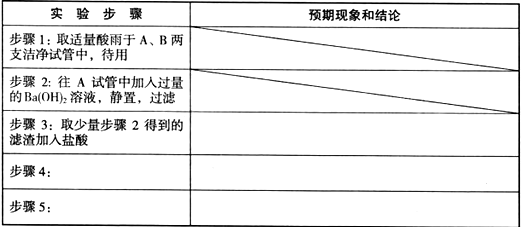
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ��װ�� | �������� | �Լ����� | ʵ������ |
| A | SO2 | | |
| B | CO2 | | |
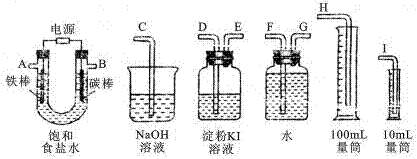
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| V/(NaOH)/mL | 0.00 | 10.00 | 18.00 | 19.80 | 19.98 | 20.00 | 20.02 | 20.20 | 22.00 | 40.00 |
| ��ҺpH | 2.87 | 4.74 | 5.70 | 6.74 | 7.74 | 8.72 | 9.70 | 10.70 | 11.70 | 12.50 |
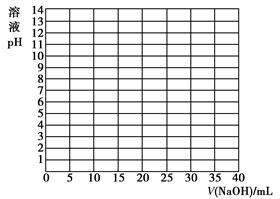
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����������� | ���͡����� |
| A�� | ͭƬ����Ũ�����У������Ա仯 | ͭ�����Ũ�����лᷢ���ۻ� |
| B�� | ��ij�Ȼ�����Һ�еμӰ�ˮ��������ɫ���� | ���Ȼ�����AlCl3 |
| C�� | ��10mlijpH=3��HA��Һ��ˮϡ�͵�100ml��������ҺpH=3.8 | HA������ |
| D�� | ��MgCl2��Һ�еμ�NaOH��Һ������pH=9ʱ����ʼ���ֳ���[��֪Mg(OH)2��Ksp=5.6��10-12] | ԭ��Һ�� c(Mg2+)=5.6��10-2mol��L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
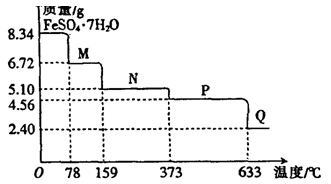
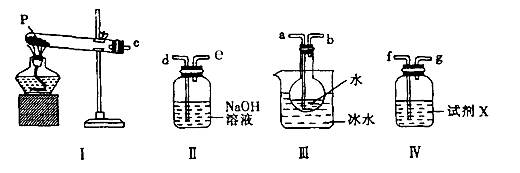
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com