
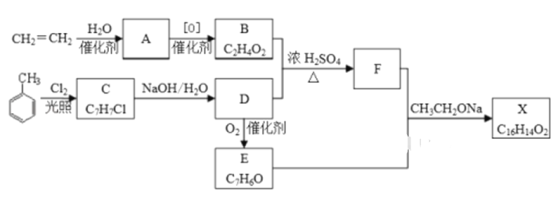
����Ŀ��������X��һ�����ϣ��ɲ�����ϩ��ױ�Ϊ��Ҫԭ�ϣ�������·�ߺϳɣ�
��֪��RX![]() ROH��RCHO+CH3COOR��
ROH��RCHO+CH3COOR��![]() RCH=CHCOOR��
RCH=CHCOOR��
��ش�
��1��C �Ľṹ��ʽ________________ ��A�й����ŵ�������__________
��2��B+D��F�Ļ�ѧ����ʽ___________________________���䷴Ӧ����Ϊ_____________
��3��X�Ľṹ��ʽ___________________
��4��D��E�Ļ�ѧ����ʽ____________________________________
��5��F�ж���ͬ���칹�壬��������������ͬ���칹����Ŀ��______�֣���д������һ��ͬ���칹��Ľṹ��ʽ________
���DZ��Ķ�Ԫȡ����
���ܷ���ˮ�⼰������Ӧ
�ۺ˴Ź���������5�����շ壬�����֮��Ϊ3��2��2��2��1
���𰸡�![]() �ǻ�
�ǻ� ![]() ȡ������������Ӧ
ȡ������������Ӧ ![]()
![]() 2
2 ![]() ��
�� ![]()
��������
���ݿ�ͼ֪CH2=CH2+H2O![]() A(CH3CH2OH)��BΪ
A(CH3CH2OH)��BΪ![]() CH3COOH;��
CH3COOH;��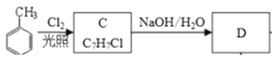 ֪CΪ
֪CΪ![]() �� DΪ
�� DΪ![]() ��������ΪE.(
��������ΪE.(![]() )��B+D
)��B+D![]() FΪ
FΪ![]() ��E+F+
��E+F+![]() X(
X(![]() )��
)��
��1���ɿ�ͼ ֪C �Ľṹ��ʽΪ
֪C �Ľṹ��ʽΪ![]() ��A ΪCH3CH2OH�����еĹ����ŵ��������ǻ����𰸣�
��A ΪCH3CH2OH�����еĹ����ŵ��������ǻ����𰸣�![]() ���ǻ���
���ǻ���
��2��BΪCH3COOH ��DΪ![]() ����B+D��F�Ļ�ѧ����ʽ
����B+D��F�Ļ�ѧ����ʽ![]() ���䷴Ӧ����Ϊȡ����Ӧ���𰸣�
���䷴Ӧ����Ϊȡ����Ӧ���𰸣� ![]() ��ȡ������������Ӧ��
��ȡ������������Ӧ��
��3��������������֪X�Ľṹ��ʽ![]() ���𰸣�
���𰸣�![]() ��
��
��4��DΪ![]() ��D
��D![]() E,����D��E�Ļ�ѧ����ʽΪ��
E,����D��E�Ļ�ѧ����ʽΪ��![]() ��
��
��5����FΪ![]() ��F�ж���ͬ���칹�壬��������������ͬ���칹����Ŀ��
��F�ж���ͬ���칹�壬��������������ͬ���칹����Ŀ��![]() ��
��![]() ��2�֡��𰸣�
��2�֡��𰸣�![]() ��
��![]() ��
��


 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������������
A. �������ӵ�ֱ���� 1��100 nm ֮�� B. �����������������
C. ���������ķ���������ۺ��Ȼ��ƵĻ����Һ D. �������ȶ����ڵ�ԭ���ǽ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ����������ֱ���������ȵ���
A. ����ƿ B. �Թ� C. ��ƿ D. ��Ͳ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵı��淽������ȷ����
A.������ˮӦ�ܷⱣ��B.ʢҺ����Լ�ƿ��������ˮ
C.�����Ľ�����Ӧ������ú����D.��̬�������ɫϸ��ƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2-��ϩ��ʯ���ѽ�IJ���֮һ���ش��������⣺
��1���ڴ��������£�2-��ϩ��������Ӧ�Ļ�ѧ����ʽΪ_____________ ����Ӧ����Ϊ_______��
��2��ϩ��A��2-��ϩ��һ��ͬ���칹�壬���ڴ�����������������Ӧ�IJ��ﲻ�������飬��A�Ľṹ��ʽΪ__________ ��A�������ܹ���ƽ���̼ԭ�Ӹ���Ϊ_________ ����A��һ�������ºϳɸ߷��ӻ�����Ļ�ѧ����ʽΪ__________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��Cʱ��0.1 mol/L�Ĵ�����Һ��H����Ũ��Ϊa mol/L��0.01 mol/L�Ĵ�����Һ��H��Ũ��Ϊb mol/L����a:b_______10������������������С������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ѫ�쵰����Է�������ԼΪ68000����֪���к�������������Ϊ0.33%����ƽ��ÿ��Ѫ�쵰�ķ�������ԭ����ԼΪ �� ��
A.5B.4C.3D.2.408��1024
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������(Na2S2O3)�����������о��й㷺Ӧ�á��ش��������⣺
I.��ҵ���ձ�ʹ��Na2SO3����ǹ����Ʊ�Na2S2O3��װ����ͼ1��
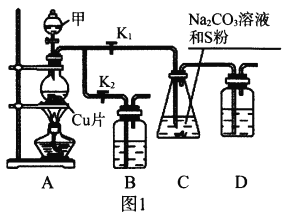
��1����K1�ر�K2����Բ����ƿ�м��������Լ��ײ����ȡ��Լ���Ϊ_________��װ��B��D��������_________��
��2��ʼ�ձ���C����Һ�ʼ��ԡ����Ȳ���Na2S2O3����Ӧ�Ļ�ѧ����ʽΪ___________________________����Ӧһ��ʱ���C��������٣���ʱ��K2���ر�K1��ֹͣ���ȣ���C�����û��������ᴿ�õ�Na2S2O3��������ʱ�ر�K1��������C����Һ�����ԡ���������Ӧ����S��_________��
��.����SO2��Na2CO3��Na2S�Ļ����Һ��ӦҲ���Ʊ�Na2S2O3������������ͼ2��

��1��װ��G��Na2CO3��Na2S��������ʵ���֮��Ϊ_________��
��2�����������Ӹ��������ӿ�˳��Ϊ��_________��g��h��_________��_________��_________��_________��d��
��.����Na2S2O3��Һ�ⶨ��ˮ��Ba2+Ũ�ȡ�
ȡ��ˮ20.00mL�������ʵ�����ȼ�������K2Cr2O7��Һ���� BaCrO4����������ϴ�Ӻ�������ϡ���ܽ⣬��ʱCrO42��ȫ��ת��ΪCr2O72�����ټӹ���KI��Һ����Cr2O72����ַ�Ӧ��Cr2O72��+6I��+14H+=3I2+2Cr3++7H2O��Ȼ����������Һ��ָʾ������0.0100mol/L��Na2S2O3��Һ���еζ���I2+2S2O32��===S4O62��+2I��������Һ_________��Ϊ�յ㡣ƽ�еζ�3�Σ�����Na2S2O3��Һ��ƽ������Ϊ18.00m����÷�ˮ��Ba2+�����ʵ���Ũ��Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������к͵ζ��У�ȡ��20��00mL��NaOH��ҺӦ��ʹ��
A.�ձ�B.��ƽC.��ʽ�ζ���D.��ʽ�ζ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com