
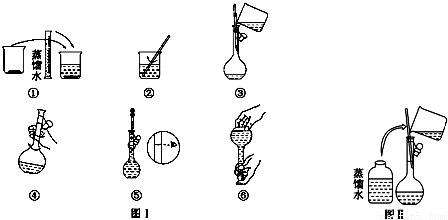
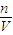 ����������������ҺŨ�ȵ�Ӱ�죮
����������������ҺŨ�ȵ�Ӱ�죮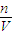 ������Һ����������������
������Һ���������������� 


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013ѧ�������ʡ�����и�����ѧ�ھ����¿���ѧ�Ծ����������� ���ͣ�ʵ����
��10�֣�ijУ��ѧʵ��С������������������480 mL 0.5 mol/L��NaOH��Һ�����ڵ���ˮ�ʼ�⡣�Իش�������⣺
(1)��С��ͬѧѡ��________ mL������ƿ��
(2)�����������ͼ����ʾ������ͼ����ʾ����Ӧ��ͼ���е�________(��ѡ����ĸ)֮�䡣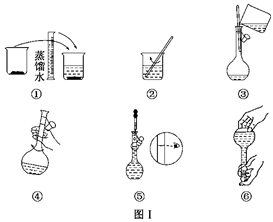
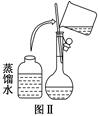
| A���ں͢� | B���ٺ͢� | C���ܺ͢� | D���ۺ͢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013ѧ�������ʡ������ѧ�ھ����¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
��10�֣�ijУ��ѧʵ��С������������������480 mL 0.5 mol/L��NaOH��Һ�����ڵ���ˮ�ʼ�⡣�Իش�������⣺
(1)��С��ͬѧѡ��________ mL������ƿ��
(2)�����������ͼ����ʾ������ͼ����ʾ����Ӧ��ͼ���е�________(��ѡ����ĸ)֮�䡣
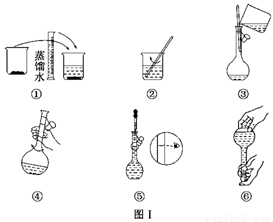
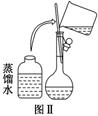
A���ں͢� B���ٺ͢� C���ܺ͢� D���ۺ͢�
(3)��С��ͬѧӦ��ȡNaOH����________g
(4)���в�������������Һ��Ũ�ȴ�С�к�Ӱ�죿
��ת������Һ��δϴ�Ӳ��������ձ���Ũ��________(�ƫ����ƫС������Ӱ�족����ͬ)��
������ƿ��ԭ������������ˮ��Ũ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
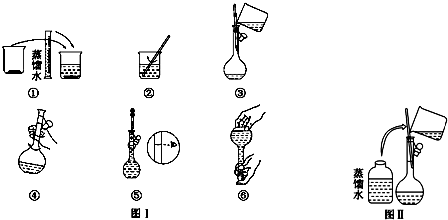
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com