

 â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
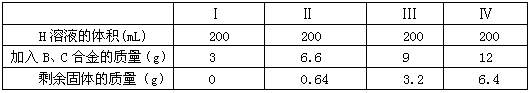
 ��2012?����һģ����֪AΪ��ѧ��ѧ�е�һ���Σ�B��CΪ�ճ������г����Ľ�����ͨ��������D��GΪ��ɫ��ζ���壮��֪�ö��Ե缫���A��Һһ��ʱ�����ֻ��C��D��E��ϡ��Һ��������֮���ת����ϵ��ͼ�����ַ�Ӧ��������ȥ����
��2012?����һģ����֪AΪ��ѧ��ѧ�е�һ���Σ�B��CΪ�ճ������г����Ľ�����ͨ��������D��GΪ��ɫ��ζ���壮��֪�ö��Ե缫���A��Һһ��ʱ�����ֻ��C��D��E��ϡ��Һ��������֮���ת����ϵ��ͼ�����ַ�Ӧ��������ȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪AΪ��ѧ��ѧ�еij���������X��YΪ�����ǽ�����X��E��F��G��J������Ϊ���壬CΪ��ɫҺ�壬B��һ���Σ������ֽ⡣����A��ʯī���缫��B��Ũ��Һ������ʣ�����ԭ��ء��й�����֮���ת����ϵ����ͼ(���ַ�Ӧ�������������ﱻ��ȥ)��

����д���пհף�
�Ž���AΪ ������a�ǽ�D��Һ��HCl���������ɣ�ԭ����
�Ʒ�Ӧ�ڵĻ�ѧ����Ϊ�� ��
��Ӧ�ݵĻ�ѧ���̣� ��
��ԭ��ط�Ӧ���У�������ӦʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�캣��ʡ�����и߿�ģ����ԣ�һ����ѧ�Ծ� ���ͣ������
��10�֣���֪AΪ��ѧ��ѧ�е�һ���Σ�B��CΪ�ճ������г����Ľ�����ͨ��
������DΪ��ɫ��ζ���壬��ɫ����G�����������ɫ����֪�ö��Ե缫���A��Һһ��ʱ�����ֻ��C��D��E��ϡ��Һ��������֮���ת����ϵ����ͼ�����ַ�Ӧ��������ȥ����
��ش��������⣺
��1��A�Ļ�ѧʽΪ ��
��2��A��Һ��Na2O2��Ӧ���ܻ�ѧ����ʽΪ ��
��3��E��ϡ��Һ��F��Һ��Ӧ�����ӷ���ʽΪ ��
��4�����100mL��A����Һһ��ʱ��Ͽ���·��ȡ���缫��������õ�������D�ڱ�״���µ����Ϊ5.6mL���������Һ��pHΪ ����������Һ������䣩
��5������100mL��A����Һ�м���10g��������B�ķ�ĩ����ֽ�����ˣ���ɵ�10.16g���壬����Һ�����ʵ����ʵ���Ũ��Ϊ ����������Һ������䣩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꺣��ʡ�����и߿�ģ����ԣ�һ����ѧ�Ծ� ���ͣ������
��10�֣���֪AΪ��ѧ��ѧ�е�һ���Σ�B��CΪ�ճ������г����Ľ�����ͨ��
������DΪ��ɫ��ζ���壬��ɫ����G�����������ɫ����֪�ö��Ե缫���A��Һһ��ʱ�����ֻ��C��D��E��ϡ��Һ��������֮���ת����ϵ����ͼ�����ַ�Ӧ��������ȥ����

��ش��������⣺
��1��A�Ļ�ѧʽΪ ��
��2��A��Һ��Na2O2��Ӧ���ܻ�ѧ����ʽΪ ��
��3��E��ϡ��Һ��F��Һ��Ӧ�����ӷ���ʽΪ ��
��4�����100mL��A����Һһ��ʱ��Ͽ���·��ȡ���缫��������õ�������D�ڱ�״���µ����Ϊ5.6mL���������Һ��pHΪ ����������Һ������䣩
��5������100mL��A����Һ�м���10g��������B�ķ�ĩ����ֽ�����ˣ���ɵ�10.16g���壬����Һ�����ʵ����ʵ���Ũ��Ϊ ����������Һ������䣩
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com