
���û�ѧ��Ӧԭ���о������������е�ʵ���������ʮ����Ҫ�����壺
�������������ϳɰ��ǻ�ѧ��ҵ�м�Ϊ��Ҫ�ķ�Ӧ�����Ȼ�ѧ����ʽ�ɱ�ʾΪ��N2(g)��3H2(g)  2NH3(g)����H����92 kJ��mol��1����ش��������⣺
2NH3(g)����H����92 kJ��mol��1����ش��������⣺
(1)ȡ1 mol N2(g)��3 mol H2(g)����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų��������ߣߣߣߣ�92 kJ(����ڡ������ڡ���С�ڡ�)��ԭ���ǣߣߣߣߣߣߣߣ��������������H������(������С�����䡱)��
(2)��֪���ֱ��ƻ�1 mol N��N����1 mol H��H����Ҫ���յ�����Ϊ��946 kJ��436 kJ�����ƻ�1 mol N��H����Ҫ���յ�����Ϊ�ߣߣߣߣߣ�kJ��
(3)N2H4����Ϊ��NH3�����е�H����NH2ȡ���IJ������������N2H4(g)Ϊȼ�ϣ�NO2Ϊ����������N2��H2O(g)��
��֪��N2(g)��2O2(g)===2NO2(g) ��H1����67��7 kJ��mol��1
N2H4(g)��O2(g)===N2(g)��2H2O(g) ��H2����534 kJ��mol��1��
��1 mol N2H4��ȫ��Ӧ���Ȼ�ѧ����ʽΪ ��
����ijǦ���ص�����������DZ�ĥ��������ͼװ�����ʵ�飬ʶ�����Ǧ���ص���������
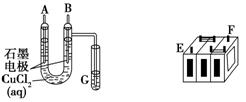
(1)��A��E��B��F����B�缫���� ����ӦʽΪ ����˵��FΪ������
��2����Ǧ���ع���ʱ���ŵ磩����E���ڵ缫�ĵ缫��ӦʽΪ�� �����ʱ�ü�����ӵ�Դ�� ��������
��3�����øõ�ص��Cu(NO3)2 ��Һ�����ⷽ��ʽΪ
����0.2mol���ӷ���ת�ƣ����������ĵ�PbO2�����ʵ����� ��Ҫ��CuSO4��Һ�ָ�ԭ���������������� ,����Ϊ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| t/s | 0 | 500 | 1000 |
| C��N2O5��/mol?L-1 | 5.00 | 3.52 | 2.48 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
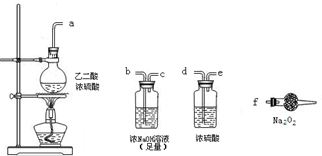
| ||
| �� |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
���û�ѧ��Ӧԭ���о������������е�ʵ���������ʮ����Ҫ�����壺
�������������ϳɰ��ǻ�ѧ��ҵ�м�Ϊ��Ҫ�ķ�Ӧ�����Ȼ�ѧ����ʽ�ɱ�ʾΪ��N2(g)��3H2(g) 2NH3(g)����H����92 kJ��mol��1����ش��������⣺
(1)ȡ1 mol N2(g)��3 mol H2(g)����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų��������ߣߣߣߣ�92 kJ(����ڡ������ڡ���С�ڡ�)��ԭ���ǣߣߣߣߣߣߣߣ��������������H������(������С�����䡱)��
(2)��֪���ֱ��ƻ�1 mol N��N����1 mol H��H����Ҫ���յ�����Ϊ��946 kJ��436 kJ�����ƻ�1 mol N��H����Ҫ���յ�����Ϊ�ߣߣߣߣߣ�kJ��
(3)N2H4����Ϊ��NH3�����е�H����NH2ȡ���IJ������������N2H4(g)Ϊȼ�ϣ�NO2Ϊ����������N2��H2O(g)��
��֪��N2(g)��2O2(g)===2NO2(g) ��H1����67��7 kJ��mol��1
N2H4(g)��O2(g)===N2(g)��2H2O(g) ��H2����534 kJ��mol��1��
��1 mol N2H4��ȫ��Ӧ���Ȼ�ѧ����ʽΪ ��
����ijǦ���ص�����������DZ�ĥ��������ͼװ�����ʵ�飬ʶ�����Ǧ���ص���������
(1)��A��E��B��F����B�缫���� ����ӦʽΪ ����˵��FΪ������
��2����Ǧ���ع���ʱ���ŵ磩����E���ڵ缫�ĵ缫��ӦʽΪ�� �����ʱ�ü�����ӵ�Դ�� ��������
��3�����øõ�ص��Cu(NO3)2��Һ�����ⷽ��ʽΪ
����0.2mol���ӷ���ת�ƣ����������ĵ�PbO2�����ʵ����� ��Ҫ��CuSO4��Һ�ָ�ԭ���������������� ,����Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡΫ���и߶�������ҵ��ѧ��һ���Ծ� ���ͣ������
���û�ѧ��Ӧԭ���о������������е�ʵ���������ʮ����Ҫ�����壺
�������������ϳɰ��ǻ�ѧ��ҵ�м�Ϊ��Ҫ�ķ�Ӧ�����Ȼ�ѧ����ʽ�ɱ�ʾΪ��N2(g)��3H2(g)  2NH3(g)����H����92 kJ��mol��1����ش��������⣺
2NH3(g)����H����92 kJ��mol��1����ش��������⣺
(1)ȡ1 mol N2(g)��3 mol H2(g)����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų��������ߣߣߣߣ�92 kJ(����ڡ������ڡ���С�ڡ�)��ԭ���ǣߣߣߣߣߣߣߣ��������������H������(������С�����䡱)��
(2)��֪���ֱ��ƻ�1 mol N��N����1 mol H��H����Ҫ���յ�����Ϊ��946 kJ��436 kJ�����ƻ�1 mol N��H����Ҫ���յ�����Ϊ�ߣߣߣߣߣ�kJ��
(3)N2H4����Ϊ��NH3�����е�H����NH2ȡ���IJ������������N2H4(g)Ϊȼ�ϣ�NO2Ϊ����������N2��H2O(g)��
��֪��N2(g)��2O2(g)===2NO2(g) ��H1����67��7 kJ��mol��1
N2H4(g)��O2(g)===N2(g)��2H2O(g) ��H2����534 kJ��mol��1��
��1 mol N2H4��ȫ��Ӧ���Ȼ�ѧ����ʽΪ ��
����ijǦ���ص�����������DZ�ĥ��������ͼװ�����ʵ�飬ʶ�����Ǧ���ص���������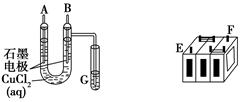
(1)��A��E��B��F����B�缫���� ����ӦʽΪ ����˵��FΪ������
��2����Ǧ���ع���ʱ���ŵ磩����E���ڵ缫�ĵ缫��ӦʽΪ�� �����ʱ�ü�����ӵ�Դ�� ��������
��3�����øõ�ص��Cu(NO3)2 ��Һ�����ⷽ��ʽΪ
����0.2mol���ӷ���ת�ƣ����������ĵ�PbO2�����ʵ����� ��Ҫ��CuSO4��Һ�ָ�ԭ���������������� ,����Ϊ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com