
��ÿ��2�֣���10�֣�������ʵ����գ�
��1����֪ij�����¿ɷ������·�ӦCH3��CH3��CH2��CH2��H2���йػ�ѧ���ļ������£�
| ��ѧ�� | C��H | C��C | C��C | H��H |
| ���ܣ�kJ/mol�� | 414.4 | 615.3 | 347.4 | 435.3 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ÿ��2�֣���10�֣�����F�Ǻϳɶ�������ҩ�������������ʵ���Ҫ�м��壬��ϳ�·�����£�
��1����Ӧ�ٵ�����Ϊ ����Ӧ�ܵ�����Ϊ ��
��2������A�ۺϿɵþ۱�Ȳ���������ṹ��ʽΪ ��
��3������C������Ϊ���ȩ��д����ͬʱ��������������һ��ͬ���칹��Ľṹ��ʽ ��
�������Ȼ�����Һ����ɫ���ڱ�����������ȡ�������۷��ӵĺ˴Ź���������4���塣
��4��������C������Ϊ����ᣬ��Ӧ�ڽ��������������ʽΪC14H15NO3����д����ṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ��Ϫһ�и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��ÿ��2�֣���10�֣�ʳƷ��ȫ��ϵ����������Ӱ��ʳƷ��ȫ�����غܶࡣ
(1)��ƫ������ϩ(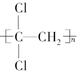 )���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������________(д�ṹ��ʽ)�����Ӿ۷�Ӧ���ɵġ�
)���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������________(д�ṹ��ʽ)�����Ӿ۷�Ӧ���ɵġ�
(2)����ֲ�����е�������[CH3(CH2)4��CH===CH��CH2��CH===CH��(CH2)7COOH]�����ܵ͡����й����������˵���У���ȷ����________��
| A������ʽΪC18H34O2 |
| B��һ���������������ᷢ��������Ӧ |
| C���ܺ�NaOH��Һ��Ӧ |
| D����ʹ����KMnO4��Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��ÿ��2�֣���10�֣�ʳƷ��ȫ��ϵ����������Ӱ��ʳƷ��ȫ�����غܶࡣ
(1)��ƫ������ϩ( )���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������________(д�ṹ��ʽ)�����Ӿ۷�Ӧ���ɵġ�
)���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������________(д�ṹ��ʽ)�����Ӿ۷�Ӧ���ɵġ�
(2)����ֲ�����е�������[CH3(CH2)4��CH===CH��CH2��CH===CH��(CH2)7COOH]�����ܵ͡����й����������˵���У���ȷ����________��
A������ʽΪC18H34O2
B��һ���������������ᷢ��������Ӧ
C���ܺ�NaOH��Һ��Ӧ
D����ʹ����KMnO4��Һ��ɫ
(3)�پ��м״�(CH3OH)�������꣬��д��Na�ͼ״���Ӧ�Ļ�ѧ����ʽ��____________________��
(4)�����̷��е����ʺ����ܵ͡�������ˮ������ղ�����__________________��
(5)�ڵ����м�������Ƶõķ�˿�ж����������յ�ˮ������������ǡ������ʵ��֤�������Ѿ�ȫ��ˮ�⣬д������������ͽ��ۣ�_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��������ѵ���и߶���ѧ�ڵ����ο��Ի�ѧ�Ծ��������棩 ���ͣ������
��ÿ��2�֣���10�֣�
ij��A 0.2 mol ����������ȫȼ�պ�����CO2��H2O��1.2 mol���Իش�
��1����A�ķ���ʽΪ_____________��
��2����ȡһ��������A��ȫȼ�պ�����CO2��H2O��3 mol������________g��A�μ��˷�Ӧ��ȼ��ʱ���ı�״���µ�����___________L��
��3������A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�����A�Ľṹ��ʽΪ__________________��
��4������A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4��������A�����еĽṹ��ʽΪ______________��д��һ�ּ��ɣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com