
���� ��1�������������̼��Ʒ�Ӧ���ɵ������������ˮ�����ʣ������ڴ���ʯ������ֹ��Ӧ���У�
�ڳ������˺�ϴ�ӣ����
���������̷���������ʯ�����ᷴӦ����������ƣ�������������һˮ�ϰ���Ӧ��������������������Һ�м���̼��立�Ӧ����̼��Ƴ��������˵õ�̼��ƣ���Һ�к��������ӣ�笠����ӣ�����笠����ӵļ��鷽���Ǻ��������Ʒ�Ӧ���ɰ������ʵ�鷽�����м��飻
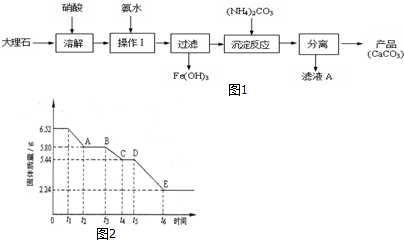
��2���ڼ��ȵ�t1ʱxCaSO4•yH2O��ʼ�ֽ⣬t1��t2ʱ��κ�t3��t4ʱ��θ�����������仯�Ƚ��٣���t5��t6ʱ��ι��������仯�ϴ�ԭ����t1��t2ʱ�����ʯ����ȥ����ˮ��t3��t4ʱ�����ȫ��ˮ����t4ʱ��ȫ����CaSO4����t5��t6ʱ����¶Ƚϸߣ�CaSO4��ʼ�ֽ⣬�����仯�ϴ�
��� �⣺��1���������̼��Ʒ�Ӧ���ɵ������������ˮ�����ʣ������ڴ���ʯ������ֹ��Ӧ���У�����ѡ�����ᷴӦ��
�ʴ�Ϊ�����������ˮ��
�����������У������롱�ò�ƷΪ̼��Ƴ������������Һ����������ʵ���������Ϊ���ˣ�ϴ�ӣ����
�ʴ�Ϊ��ϴ�ӣ����
�۴���ʯ�����ᷴӦ����������ƣ�������������һˮ�ϰ���Ӧ��������������������Һ�м���̼��立�Ӧ����̼��Ƴ��������˵õ�̼��ƣ���Һ�к���������Ϊ�����ӣ�笠����ӣ�����笠����ӵļ��鷽���Ǻ��������Ʒ�Ӧ���ɰ���������ʪ��ĺ�ɫʯ����ֽ����֤�����������ɣ�
�ʴ�Ϊ��NH4+���ռ
��2��������ͼ�����������������0-t1ʱ����ޱ仯��˵������δ��Ӧ�����ȵ�t1ʱ������������ʼ��С�����Ըþ��忪ʼ������ѧ�仯��ʱ����t1��
�ʴ�Ϊ��t1��
���ڼ��ȵ�t1ʱxCaSO4•yH2O��ʼ�ֽ⣬t1��t2ʱ��κ�t3��t4ʱ��θ�����������仯�Ƚ��٣���t5��t6ʱ��ι��������仯�ϴ�ԭ����t1��t2ʱ�����ʯ����ȥ����ˮ��t3��t4ʱ�����ȫ��ˮ��t4��t5ʱ��ι���Ļ�ѧʽΪCaSO4��
�ʴ�Ϊ��CaSO4��
��t3��t4ʱ�����ȫ��ˮ����m��H2O��=6.52g-5.44g=1.08g��m��CaSO4��=5.44g��n��H2O��=$\frac{1.08g}{18g/mol}$=0.06mol��n��CaSO4��=$\frac{5.44g}{136g/mol}$=0.04mol������n��CaSO4����n��H2O��=x��y=0.04��0.06=2��3����ѧʽΪ��2CaSO4•3H2O��tl��t2ʱ��ι��������仯������ˮ������=6.52g-5.80g=0.72g��n��H2O��=$\frac{0.72g}{18g/mol}$=0.04mol����������Ϊ5.80g��6.52g����ƹ������ʵ���=$\frac{6.52g}{326g/mol}$=0.02mol����1mol2CaSO4•3H2Oʧˮ2mol����Ӧ�Ļ�ѧ����ʽΪ��2CaSO4•3H2O$\frac{\underline{\;\;��\;\;}}{\;}$2CaSO4•H2O+2H2O��
�ʴ�Ϊ��2CaSO4•3H2O$\frac{\underline{\;\;��\;\;}}{\;}$2CaSO4•H2O+2H2O��
���� ���⿼����̼����Ʊ����̷����жϣ���ʯ����ȵı仯ͼ����Ŀ�ѶȽϴ�ע�����ͼ��������仯�жϿ��ܷ����ķ�Ӧ��


 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | ��Է������� | �е㣨�棩 | �ܶȣ�g•cm-3��20�棩 | �ܽ��� |
| ������ | 100 | 161.1 | 0.9624 | ������ˮ���� |
| ����ͪ | 98 | 155.6 | 0.9478 | ����ˮ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C2H2 | B�� | C2H4 | C�� | C3H8 | D�� | C2H6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 44g14C1802�����е�������Ϊ28NA | |
| B�� | 32gCH4���������ۼ���Ϊ8NA | |
| C�� | 7.8gNa2O2����������������Ϊ0.4NA | |
| D�� | 17gOH-�����еĵ�����Ϊ9NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��Z��ʾ�Ļ�ѧ��Ӧ����Ϊ0.016 mol•L-1•s-1 | |
| B�� | ���º����£�ͨ��Ne��������ѹǿ��Y��Ũ�Ȳ���С | |
| C�� | ���º����£��ٳ���һ������Z�����´ﵽƽ��ʱ��X�����ʵ���������С | |
| D�� | ��ƽ���ʵ�ʷų�������Ϊb kJ����b=a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ï�������еĵ�����������˭һ����Ȼ�������ǹ�ѧ�����뽺��֪ʶ�� | |
| B�� | ��������ͷ���ijЩ����Ԫ�ػ�ѧ���ʵ�չ�� | |
| C�� | ��������������������ЧӦ�������⻯ѧ���������γɶ��뵪���������� | |
| D�� | CO2�ϳɾ�̼�����ɽ������ϣ���ʵ�֡�̼����ѭ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
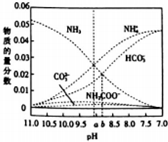 �����仯������������������������Ҫ���ã�
�����仯������������������������Ҫ���ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | HF�ĵ���ʽΪ��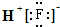 | B�� | HClO�Ľṹʽ��H-Cl-O | ||
| C�� | CO2�ĵ���ʽΪ��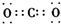 | D�� | Cl-�Ľṹʾ��ͼ��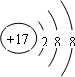 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com