
 ��������0.1mol?L-1������ҺNaOH��NH3?H2O��CH3COOH��HCl��NH4HSO4��
��������0.1mol?L-1������ҺNaOH��NH3?H2O��CH3COOH��HCl��NH4HSO4��| c(H+) |
| c(CH3COOH) |
| c(H+) |
| c(CH3COOH) |
| 10-3 |
| 0.1 |
| 10-14 |
| 10-3 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Cl- | ||
B��HCO
| ||
| C��Na+ | ||
D��NO
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧ�� | H-H | N-H | N��N |
| ����/kJ?mol-1 | 436 | a | 945 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1��ij��Ӧ�����е������仯��ͼ��ʾ��д���÷�Ӧ���Ȼ�ѧ����ʽ
��1��ij��Ӧ�����е������仯��ͼ��ʾ��д���÷�Ӧ���Ȼ�ѧ����ʽ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼװ�ã�����������ʢ�����з�̪��Һ��NaCl������Һ��A��BΪ���ʯī�缫����ͨS1һ��ʱ��۲쵽A������Һ��죬�������������������ɣ����Ͽ�S1����ͨS2���۲쵽��������ָ�뷢��ƫת����������������������Һ��δ����缫����ش�
��ͼװ�ã�����������ʢ�����з�̪��Һ��NaCl������Һ��A��BΪ���ʯī�缫����ͨS1һ��ʱ��۲쵽A������Һ��죬�������������������ɣ����Ͽ�S1����ͨS2���۲쵽��������ָ�뷢��ƫת����������������������Һ��δ����缫����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��3�� | B��4�� | C��5�� | D��6�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����NaOH |
| B��ͨ��H2S���� |
| C������ |
| D������Na2S���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CO2�Ľṹʽ��O-C-0 |
B�������Ƶĵ���ʽ�� |
C��CCl4���ӵı���ģ�ͣ�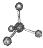 |
D�����ʯ�Ľṹģ�ͣ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com