
�Ȼ�ѧ����ʽ����д
����֪0.4 molҺ̬�º�����H2O2��Ӧ�����ɵ�����ˮ�������ų�256.65 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
����֪��ѧ����ʽ��H2(g)+1/2O2(g) ��H2O(g)���÷�Ӧ�Ļ��Ϊ167.2 kJ/mol�����淴Ӧ�Ļ��Ϊ409.0 kJ/mol��д���÷�Ӧ���Ȼ�ѧ����ʽ
����֪1mol����ת�����Ӧ�������ĺ���ʱ�ų�29��2KJ������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
�� ��֪S(s) + O2(g)  SO2(g) ����4NA������ת��ʱ���ų�297��23 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
SO2(g) ����4NA������ת��ʱ���ų�297��23 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��1��CH4��ȫȼ������CO2��H2Oʱ���ų�Q kJ��������д���˷�Ӧ���Ȼ�ѧ����ʽ ��
�ƾ�ȼ�յĻ�ѧ����ʽΪ��C2H6O(l)+3O2(g)��2CO2(g)+3H2O(l)����ȫȼ��һ��������ˮ�ƾ����ų�������ΪQ kJ������ȫ�������ɵ�CO2������8 mol/L��NaOH��Һ50 mLʱǡ���������Ρ�д���˷�Ӧ���Ȼ�ѧ����ʽ ��
��N2H4(l) + 2H2O2(l) == N2(g)+4H2O(g)����H= ��641.6KJ/mol��1
��H2O(g) �� H2(g)+1/2O2(g) ����H= ��409.0KJ/mol��1
��P4��S�����ף�== 4P��S�����ף� ��H = ��29.2KJ/mol��1
�� S(s) + O2(g)  SO2(g); ��H = ��297.23KJ/mol��1
SO2(g); ��H = ��297.23KJ/mol��1
�� CH4(g) + 2O2(g) == CO2(g) +2H2O(l) ; ��H= ��16Q kJ��mol-1
��C2H6O(l)+3O2(g)��2CO2(g)+3H2O(l) ; ��H= ��10Q kJ��mol-1


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����( )
A������ƿ���ձ�����Ͳʹ��ǰ����Ҫ��©��ϴ�������
B����Һʱ�����ȴ�Һ©���϶˵IJ��������ٽ��²�Һ����¿ڷų�
C��������ͭ��Һ�������д�����������ʱ��ֹͣ���ȣ���ɵõ���������
D������һ�����ʵ���Ũ����Һʱ��������ƿ��Һ����ڿ̶���ʱ���ý�ͷ�ι���������Һ�弴��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������Ҫ0.1 mol/L NaOH��Һ450 mL��0.5 mol/L������Һ500 mL��������������Һ����������ش��������⣺
����ͼ��ʾ�������У�������Һ�϶�����Ҫ����__________________________ (�����)������������Һ�����õ��IJ��������� (����������)��
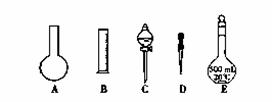
������0.1 mol/L NaOH��Һʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ� ��
A��������ƿ�ǽ�����ҡ��
B�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
C������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1~2cm��
D����30mLˮϴ���ձ�2~3�Σ�ϴ��Һ��ע������ƿ
E�����ܽ������������Һ�ز�����ע��500mL������ƿ��
F��ȷ��ȡ���������������ƹ������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ�����ܽ�
������0.1 mol/L NaOH��Һʱ����ʵ����������������ȷ��������ʱ��������ƿ�̶��ߣ���������ҺŨ�� 0.1 mol/L(����ڡ��������ڡ���С�ڡ�)��
������0. 5 mol/L������Һ500 mLʱ��������������Ϊ98%���ܶ�Ϊ1.84 g/cm3��Ũ��������Ϊ mL(����������һλС��)��
������0.5 mol/L������Һʱ����ʵ����������������ȷ��������Ͳ��ȡŨ����ʱ���ӿ̶��ߣ���������ҺŨ�� 0.5 mol/L(����ڡ��������ڡ���С�ڡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���в���ȷ���ǣ� ��
A��X+Y��M+NΪ���ȷ�Ӧ������֪X��Y��������һ������M��N��������
B��1mol SO2�ļ����ܺʹ���1mol���1 mol�����ļ���֮��
C����C(ʯī)��C(���ʯ)����H= +1.9 kJ��mol��1��֪�����ʯ��ʯī������
D���������������������ֱ���ȫȼ�գ�ǰ�߷ų���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͬ�������£�ͬ�ֹ�����Ƭ�ֱ����������ʻ�ϣ���ѧ��Ӧ����������(����)
A��0.1mol��L��1������15mL B��0.2mol��L��1������12mL
C��0.15mol��L��1��������Һ8mL D��18mol��L��1��Ũ����15mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ������2mol/L��Na2CO3��Һ950mL������ʱӦѡ�õ�����ƿ�Ĺ��ͳ�ȡNa2CO3�������ֱ��ǣ� ��
ʵ������2mol/L��Na2CO3��Һ950mL������ʱӦѡ�õ�����ƿ�Ĺ��ͳ�ȡNa2CO3�������ֱ��ǣ� ��
A��950mL��201.4g B��1000mL��212g C��������572g D��500mL��106g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ��С��ѧ�����տα�ʵ��Ҫ����50mL0.5mol/L������50mL0.5mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų������������к��ȣ�����˵����ȷ�ǣ�������
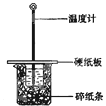
| �� | A�� | ʵ�������û��������ʧ |
| �� | B�� | ͼ��ʵ��װ��ȱ�ٻ��β�������� |
| �� | C�� | �ձ���������ֽ���������ǹ̶�С�ձ� |
| �� | D�� | ������60mL0.50mol/L�����50mL0.5mol/L��NaOH��Һ���з�Ӧ����������˵�����к��Ȳ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д�����з�Ӧ�����ӷ���ʽ��
��ϡ������NaOH��Һ��Ӧ�����ӷ���ʽ��
________________________________________________________________________
������ϡ������CaCO3��Һ��Ӧ�����ӷ���ʽ��
________________________________________________________________________
��п��ϡ���ᷴӦ�����ӷ���ʽ��
________________________________________________________________________
��ϡ���������������Һ��Ӧ�����ӷ���ʽ��
�ݵ���������ʵ���Ũ�ȵ�̼��������Һ������������Һ��Ӧ�����ӷ���ʽ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com