
(13��) ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���塣B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1��18���еĵ�7��Ԫ�ء�D��ԭ��������EС5��D��B�γɵľ����侧���ṹ��ͼ��ͼ��С�����D���������B��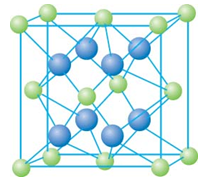
��ش�
(1)AԪ�ص�������________________��
(2)BԪ�صĹ����ʾʽ��________________��C��ԭ�ӽṹʾ��ͼ��________________��B��A�γɵĻ������C��A�γɵĻ�����е�ߣ���ԭ����__________________________________________________________��
(3)E����Ԫ�����ڱ��е�________���ڣ���________���Ԫ�أ���Ԫ��������________������Ԫ�����ڱ��е� ������Ԫ�ط����������ģ�2�����ӵĵ����Ų�ʽΪ________________��
(4)��ͼ�п��Կ�����D��B�γɵ����ӻ�����Ļ�ѧʽΪ________________�������ӻ����ᄃ����ܶ�Ϊa g?cm��3�����������________________(ֻҪ���г���ʽ)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(13��) ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���塣B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1��18���еĵ�7��Ԫ�ء�D��ԭ��������EС5��D��B�γɵľ����侧���ṹ��ͼ��ͼ��С�����D���������B��
��ش�
(1)AԪ�ص�������________________��
(2)BԪ�صĹ����ʾʽ��________________��C��ԭ�ӽṹʾ��ͼ��________________��B��A�γɵĻ������C��A�γɵĻ�����е�ߣ���ԭ����__________________________________________________________��
(3)E����Ԫ�����ڱ��е�________���ڣ���________���Ԫ�أ���Ԫ��������________������Ԫ�����ڱ��е� ������Ԫ�ط����������ģ�2�����ӵĵ����Ų�ʽΪ________________��
(4)��ͼ�п��Կ�����D��B�γɵ����ӻ�����Ļ�ѧʽΪ________________�������ӻ����ᄃ����ܶ�Ϊa g•cm��3�����������________________(ֻҪ���г���ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009��2010ѧ����������������߶���ѧ��ѧ����ĩ��� ���ͣ�������
(12��)
����֪�����£�AgCl��Ksp��1.8��10-10��AgBr��Ksp��4.9��10-13��
��1������AgCl������Һ�У�
�ټ���AgNO3���壬��c(Cl-) ����������С�����䡱����ͬ����
�����ļӸ����AgCl���壬��c(Ag+) ��
�����ļӸ����KBr���壬��c(Ag+) ��c(Cl-) ��
��2���й������ε��ܶȻ����ܽ��������������������ȷ���� ��
| A�������ܵ���ʷ��봿ˮ�У��ܽ�ﵽƽ��ʱ�������¶ȣ� Ksp һ������ |
| B�����������ε���ʣ�����KspС���ܽ��Ҳһ��С |
| C�������ε���ʵ�Ksp���¶��й� |
| D����AgCl������Һ�м���������ˮ��ʹAgCl�ٴδﵽ�ܽ�ƽ�⣬AgCl��Ksp���䣬���ܽ��Ҳ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��Ȫ�ݽ����и߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ������
(13��)��1��A��B��DΪ������Ԫ�أ��������Ϣ�ش����⣺
|
Ԫ�� |
A |
B |
D |
|
���ʻ�ṹ��Ϣ |
��ҵ��ͨ������Һ̬��������䵥�ʣ���������ȼ |
��̬�⻯���ˮ��Һ�Լ��� |
ԭ�����������Ӳ㣬�������ڱ������а뾶��С |
�ٵ�һ�����ܣ�A B���>��������������<��������̬Dԭ�ӵĵ����Ų�ʽΪ ��
��B��D�ɹ��ۼ��γɵ�ij������BD��2200�濪ʼ�ֽ⣬BD�ľ�������Ϊ ��
��2����չú��Һ�����������롰ʮ����滮�����п�Ժɽ��ú��������úҺ�������ĸ�Ч�����з���Ŀȡ�û�����չ����֪��ú������ת��Ϊһ����̼�����������ڴ��������ºϳɼ״���CH3OH��,�Ӷ�ʵ��Һ����
��ij��ͭ���ӵ����ӽṹ����ͼ��ʾ��

�ڸ������ڲ������������������У� ������ĸ����
A�����Ӽ� B.���Լ� C.�Ǽ��Լ� D.��λ�� E.���»��� F.���
��úҺ����ü״�,�پ����õ���Ҫ��ҵԭ�ϼ�ȩ(HCHO)���״��ķе�Ϊ65�棬��ȩ�ķе�Ϊ��21�棬���߾�������ˮ���״��ķе�ȼ�ȩ������Ϊ�״����Ӽ���������������ȩ���Ӽ�û��������״��ͼ�ȩ������ˮ������Ϊ���Ǿ����Ժ�ˮ�γɷ��Ӽ����������˵����ȩ���Ӽ�û�������ԭ���� ��
�ۼ״������У�����sp3�ӻ���ԭ���� ����ȩ��H2�����ӳɷ�Ӧ��������1mol�״�ʱ�����ѵġǼ�����ĿΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�ϸԸ���ѧ�߶���һѧ�����п��Ի�ѧ�Ծ���ѡ�ޣ� ���ͣ�������
(9��)����A��B������������һԪ���Ļ����0.3mol��������Ϊ13.8g����֪A��B̼ԭ������������4����A<B��
�Ż������A�ķ���ʽΪ_______________��
����n(A)��n(B)=1��1ʱ��B�Ľṹ��ʽΪ____________________________��
����n(A)��n(B)��1��1ʱ��B�ķ���ʽΪ_________________��n(A)��n(B)=___________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com