
����Ŀ����֪ͭ�������A�ṹ��ͼ����ش��������⣺
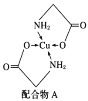
��1��д����̬Cu����Χ�����Ų�ʽ��__��
��2�����就�������(H2NCH2COO-)���ȷֽ�ɲ���CO2��N2��N2��������������Ŀ֮����__��N2O��CO2��Ϊ�ȵ����壬��N2O�Ŀռ�ṹΪ___��
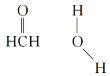
��3����Cu���£��״��ɱ�����Ϊ��ȩ����ȩ������HCO�ļ���__(����������������������С����)120������ȩ����ˮ�γ������������ͼ�б�ʾ����___��
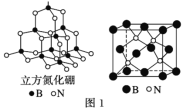
��4��������������ͼ1����ʯ�ṹ���ƣ��dz�Ӳ���ϡ���������������B��N��������ԭ����֮��Ϊ__��
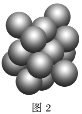
��5��Cu����Ķѻ���ʽ��ͼ2��ʾ����Cuԭ�Ӱ뾶Ϊa��������Cuԭ�ӵ���λ��Ϊ___������Ŀռ�������Ϊ__��(��֪��![]() ��1.4)
��1.4)
���𰸡�3d104s1 1��2 ֱ���� ���� 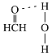 4��1 12 74.76%
4��1 12 74.76%
��������
(1)CuΪ29��Ԫ�أ�Cuԭ�Ӻ�����29�����ӣ����ݺ�������Ų����ɿ�֪��̬ͭԭ�ӵĵ����Ų�ʽΪ[Ar] 3d104s1����Χ�����Ų�Ϊ3d104s1��
(2)N2�ĽṹʽΪN��N�������к�1��������2����������N2���������� ����Ŀ֮��Ϊ1��2���ȵ�����Ľṹ���ƣ�������̼������Cԭ��Ϊsp�ӻ�������Ϊֱ���Σ�����N2O�Ŀռ�ṹΪֱ���Σ�
(3)��ȩ��C��sp2�ӻ�����ƽ�������Σ�Cԭ��������������λ�ã�Oԭ�Ӻ��еŶԵ�����ɼ����Ӷ�֮����ų������ڳɼ����Ӷ�֮����ų������ʼ�ȩ������HCO�ļ��Ǵ���120����ˮ�����к�O��H��������Oԭ�ӵ�ԭ�Ӱ뾶С���縺�ԴԹ��õ��ӶԵ���������ǿ��ʹˮ��������ԭ�Ӽ�����Ϊ��¶�����ӣ��ʼ�ȩ�����е�Oԭ�Ӻ�ˮ�����е�Hԭ�����γ�������������ʾΪ ��
��
(4)�þ�����Nԭ���ھ����ڲ���������4��ÿ��Nԭ���γ�4��B��N����B��N������Ϊ16��Bԭ��λ�ھ�����������ģ�����Ϊ![]() ����B��N����Bԭ�Ӹ���֮��Ϊ16��4=4��1��
����B��N����Bԭ�Ӹ���֮��Ϊ16��4=4��1��
(5)Cu����Ķѻ���ʽ�������������ܶѻ���������λ����12����ͼ��֪��������Խ����ϵ�����ͭԭ�����У�������Խ��߳���4a�����ݼ��ι�ϵ��֪��������ⳤ=2![]() a���������ΪV=(2
a���������ΪV=(2![]() a)3��1��Cu�������
a)3��1��Cu�������![]() ��a3��������Cuԭ�ӵĸ���=8��
��a3��������Cuԭ�ӵĸ���=8��![]() ��6��
��6��![]() =4�����Ծ���Ŀռ�������Ϊ
=4�����Ծ���Ŀռ�������Ϊ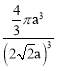 ��100%=74.76%��
��100%=74.76%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ŀǰ��ҵ�ϴ����л���ˮ��һ�ַ����ǣ��ڵ��ں�pH��Mn2��Ũ�ȵķ�ˮ�м���H2O2��ʹ�л����������⡣��������¶Ա�ʵ�飨ʵ���������±�)��ʵ�����л���RŨ����ʱ��仯�Ĺ�ϵ����ͼ��ʾ������˵����ȷ����
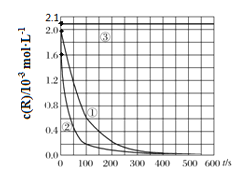
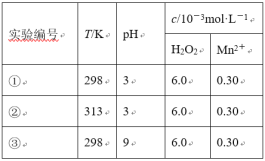
A.313Kʱ����0~100s���л���R�����ƽ������Ϊ��0.014 mol��L-1��s-1
B.�ԱȢ٢�ʵ�飬���Եó��¶�Խ��Խ�������л���R�Ľ���
C.�ԱȢ٢�ʵ�飬���Է���������ʵ�����л���R�Ľ���ٷ��ʲ�ͬ
D.ͨ����������ʵ�飬����ʹ��Ӧֹͣ��������Ӧ��ϵ�м���һ������NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
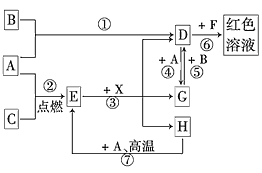
����Ŀ�������£�A�dz����Ľ������ʡ�����B�ǻ���ɫ���塢����C����ɫ���塣�ں��ʷ�Ӧ�����£����ǿ��������ͼ���з�Ӧ��E��Һ����ɫ��Һ��F�ǵ���ɫ��Һ��B��C��Ӧ������ɫ���档��ش�:
(1)A��_________��B��_________��C��_________![]() ��д��ѧʽ
��д��ѧʽ![]() ��
��
(2)��Ӧ�ٵĻ�ѧ����ʽ ______________________________________��
(3)��Ӧ�۵����ӷ���ʽ _____________________________________��
(4)��Ӧ�ܵ����ӷ���ʽ ______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
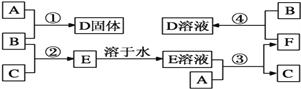
����Ŀ��A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ������£�AΪ���壬B��C��Ϊ���塣D��E��F��G��H��X��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�塣����֮���ת����ϵ��ͼ��ʾ(����ijЩ��Ӧ�����Ͳ��ַ�Ӧ������ȥ)��
��1���ڷ�Ӧ�١����У�������������ԭ��Ӧ����________(����)��
��2��д���۵����ӷ���ʽ��________________
��3����Ӧ�ߵĻ�ѧ����ʽΪ____���÷�Ӧ��ÿ����0.3 mol��A����ת�Ƶ���_________mol���ڽ������ʱ�Ĺؼ�һ����____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ���Ũ�ȵ�CuSO4��NaCl�������Ϻ���ʯī�缫���е�⣬�������У���ҺpH��ʱ��t�仯��������ͼ��ʾ��������˵���������
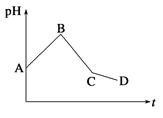
A������������Cl2��������O2�������Ȳ���Cu��������H2
B��AB������ֻ����Cl2������ֻ����Cu
C��BC�α�ʾ����������H���ŵ������H2
D��CD���൱�ڵ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ�����ã����������һ����ɫ���壬Ϊ�˽�ͭ�ڿ����еĸ�ʴ�����ij��ѧ��ȤС���ռ�����ͭ���������ɫ�������̽��������������Ϻ������ɫ���ʿ�����ͭ��̼���Ρ�
��С��ͬѧ������ͼװ�ý���ʵ��(���ּг�������)��
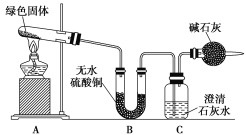
�ٶ��Թ��ڵ���ɫ������м��ȣ�����ȫ�ֽ⣬�۲쵽Aװ������ɫ������ɺ�ɫ��Bװ������ˮ����ͭ�����ɫ��Cװ���г���ʯ��ˮ����ǡ�
��ȡ�������Ⱥ����ɵĺ�ɫ�������Թ��У�����ϡ���ᣬ�۲쵽��ɫ�������ܽ⣬��Һ�����ɫ��
��ȡ����������ɫ��Һ���Թ��У�����һ���ྻ����˿���۲쵽��˿�����к�ɫ����������
��ش��������⣺
(1)��ɫ�����к��е�Ԫ����________________________________________________��
(2)���Ⱥ��Թ���ʣ��ĺ�ɫ������_________________________________________________��
(3)�������ɫ������һ�ִ�������仯ѧʽ������______________�����ȷֽ�Ļ�ѧ����ʽΪ_______________________________________________________________________________________��
(4)����ʵ�鲽����еķ�Ӧ�����ӷ���ʽΪ________________________________________��
(5)ʵ��װ�����ĸ���ܵ�������___________________________________________________��
(6)�����B��C��װ�öԵ����ܷ�ﵽʵ��Ŀ��______(������������������)��Ϊʲô��_____________________________________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������й���ѧ���ڡ���������־���߷�����һƪ������������Һ̬������������̫��ȼ���������ڿ�������ɫҺ̬ȼ�ϡ�ijģ�����˹���Ҷ������Һ̬�������绯ѧʵ��װ����ͼ��ʾ����װ���ܽ�H2O��CO2ת��ΪO2��ȼ�������(CH3)2CHOH������˵����ȷ����

A.��װ�ý���ѧ��ת��Ϊ���ܺ͵���
B.a�缫��ӦΪ3CO2+ 16H+- 18e-= (CH3)2CHOH + 4H2O
C.��װ�ù���ʱ��H+��b������a����Ǩ��
D.������ɷ���������Ӧ����ԭ��Ӧ��ȡ����Ӧ�;ۺϷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������֮�������·�Ӧ��ϵ��
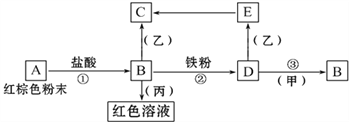
��֪��Eת����C�������ǣ��Ұ�ɫ����Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ��
�ش�
��1�� д���������ʵĻ�ѧʽ��A______B______D________��_______��_______��__________
��2�� д��E��C��Ӧ�Ļ�ѧ����ʽ��_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������棬��2011��5��1���𣬽�ֹ��������������ӹ�������(CaO2)��ʳƷ���Ӽ���CaO2��Na2O2�ڽṹ���������кܶ����Ƶĵط���������������⣺
��1��CaO2����____(����ӻ�������ۻ����)�������ʽΪ_____�������������Ӹ�����Ϊ______��
��2��CaO2��ˮ��Ӧ�Ļ�ѧ����ʽΪ______������1mol�������壬ת�Ƶĵ�����Ϊ__________��(��NA���������ӵ�����)
��3��CaO2�������̼��Ӧ�Ļ�ѧ����ʽΪ_________���÷�Ӧ����____________��
A���û���Ӧ B��������ԭ��Ӧ C�����ֽⷴӦ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com