
����Ŀ��ij��ѧ��ȤС��Ϊ��̽��������ij�ǽ����������γɵ�δ֪����ijɷ֣�������ͨ�����ʯ��ˮ�����ֳ���ʯ��ˮ����ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������롣
[�������]
����1��������ΪCO2��
����2��������ΪSO2��
����3��______________________________________________________��
Ϊ����֤�²⣬��С�����ʵ�����̽����
[ʵ��̽��]
��С��ͬѧ����ͼ��ʾװ�ã��������a��ͨ�롣
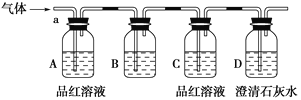
(1)B��Ӧ��װ����________�Լ�(����)��
A��NaCl��Һ B������KMnO4��Һ C������ D������ʯ��ˮ
(2)A��Ʒ����Һ��������________________________________________________��
(3)D�г���ʯ��ˮ��������______________________________________________��ͨ����ʵ�飬��С��ͬѧ�۲쵽��������ʵ������A��Ʒ����Һ��ɫ����C��Ʒ����Һ����ɫ ��D�г���ʯ��ˮ�����
[����]
(4)����������С��ͬѧȷ�ϸ�����ijɷ�Ϊ��________________________________��
���𰸡� ������ΪCO2��SO2�Ļ������ B ��֤����������Ƿ���SO2 ��֤����������Ƿ���CO2 CO2��SO2�Ļ������
��������
����ʵ�鷽����������ۣ����ݲ���1�Ͳ���2���ó�����3����������CO2��SO2�Ļ�����壻��1��SO2��CO2����ʹ����ʯ��ˮ����ǣ�SO2��ʹƷ����Һ��ɫ��CO2����ʹƷ����Һ��ɫ���������֤SO2�Ĵ��ڣ�ͨ��Ʒ����Һ�����Ʒ����Һ��ɫ��˵������SO2��������Ȼ������SO2�Ļ�ԭ�ԣ�����������ȥSO2����ͨ�����Ը��������Һ����ˮ��Ȼ����ͨ��Ʒ����Һ������SO2�Ƿ���ȫ���������ͨ�����ʯ��ˮ�����Bװ��Ӧʢ�����Ը��������Һ����B��ȷ��A��Ʒ����Һ�������������Ƿ���SO2��C��Ʒ����Һ������SO2�Ƿ���ȫ��������4��D�г���ʯ��ˮ�������Ǽ���ԭ����������Ƿ���CO2����5�����ݣ�4���Т�A��Ʒ����Һ��ɫ��˵��ԭ�����к���SO2������Ʒ����Һ����ɫ��˵��SO2��ȫ����ȥ����D�г���ʯ��ˮ����ǣ�˵��ԭ�����к���CO2����˸�����ijɷ���SO2��CO2�Ļ�����塣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȼ�ϵ���ڹ���ʱ�����ϴ�������뻹ԭ������������ͬʱ���缫��Ӧ���ﲻ���ų���ء�����4��ȼ�ϵ�صĹ���ԭ��ʾ��ͼ�����������ķ�Ӧ����Ϊˮ���ǣ� ��
A. ����������ȼ�ϵ�� B. ����ȼ�ϵ��
B. ����ȼ�ϵ��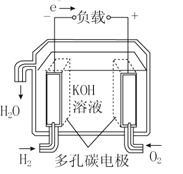 C. ���ӽ���Ĥȼ�ϵ��
C. ���ӽ���Ĥȼ�ϵ��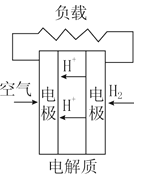 D. ������ȼ�ϵ��
D. ������ȼ�ϵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�����ķ����У�����ԭ�Ӷ�����ͬһƽ�����( )
A. ������ B. �ױ� C. ���� D. ����ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z�������嶼�ܶԴ��������Ⱦ���ڹ�ҵ�϶������ü�Һ���ա���֪X�ǻ�ʯȼ��ȼ�ղ���֮һ�����γ��������Ҫ���ʣ�Y��һ�ֵ��ʣ�����ˮ��Һ����Ư�����ã�Z�����Ṥҵ������β���е��к�����֮һ������ˮ��Ӧ����д���������ʷ�Ӧ�Ļ�ѧ����ʽ��
(1)X��һ����������������Ӧ____________________________________��
(2)Y������������Һ�ķ�Ӧ______________________________________��
(3)Z��ˮ�ķ�Ӧ________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʯ�����ѽ��� A ��Ŀǰ��ҵ������A����Ҫ;����ͼ�е� N �Ǹ߷��ӻ������ A �� 1��3 һ����ϩΪԭ�Ϻϳ� N ���������£�

(1)D ���ʵ�����Ϊ_____��B��C �Ļ�ѧ��Ӧ����ʽΪ_____________��
(2)G �к��������ŵ�����Ϊ_____________���� G��H �ķ�Ӧ����Ϊ_________��
(3)�ڴ��������£�E �� M �����ʵ��������ۺϷ�Ӧ��N �Ľṹ��ʽΪ__________��
(4)E �ж���ͬ���칹�壬��������������ͬ���칹�������______�֡�
�ٿ��� Na2CO3 ��Һ��Ӧ���ڷ�����ֻ��һ����״�ṹ��
(5)��Ȼ���������ϩ�ľۺ���䵥���� CH2=C(CH3)CH=CH2�����������ϩΪԭ��(�������Լ���ѡ)������Ʊ� �ĺϳ�·�ߣ���д���ϳ�·������ͼ_____��
�ĺϳ�·�ߣ���д���ϳ�·������ͼ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ���������ֵ������˵����ȷ����
A. ���س�ѹ�£�36g18O2��������������Ϊ16NA
B. 4.6 gNO2��N2O4�Ļ��������������ԭ����Ϊ0.1 NA
C. ��״���£�2.24 L �����еķ���������0.1 NA
D. �ڹ���������ˮ�ķ�Ӧ�У�ÿ����0.1mol�������ƣ�ת�Ƶ��ӵ���ĿΪ0.2 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȥSiO2�л��е�������ʯ�Һ�ʯ��ʯ�������²������� ��
�ټ�ˮ�ܽ� �ڼ�ˮ��� �۹��� �ܼ�ϡHCl��
A.�٢�
B.�ܢ�
C.�ݢ�
D.�ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ָ����ѧ������ȷ������ ��
A. NaHCO3ˮ������ӷ���ʽ��HCO3-+H2O = CO32-+H3O+
B. Ba(OH)2�ĵ��뷽��ʽ��Ba(OH)2![]() Ba2++2OH-
Ba2++2OH-
C. NaHSO4��ˮ�еĵ��뷽��ʽ��NaHSO4= Na++H++SO42-
D. Na2Sˮ������ӷ���ʽ��S2-+2H2O = H2S+2OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о���ѧϰС��ͬѧΪ��̽��������ͬ���¶Ⱥ�ѹǿ�¡���ͬ������κ����嶼������ͬ��Ŀ�ͷ��ӡ����������������ʵ��װ�ò���¼���ʵ�����ݡ�ʵ��װ�ã�
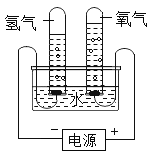
����ʵ�����ݣ�
�¶� | ѹǿ | ʱ�� | ˮ����H2O������ | H2��� | O2��� |
30�� | 101 kPa | 0 | 300 g | 0 | 0 |
30�� | 101 kPa | 4���� | 298.2 g | 1.243 L |
��ش��������⣺
(1)4����ʱH2��O2���ʵ����ֱ���__________mol��___________mol��
(2)���¶��£�����Ħ�������________________��
(3)������H2O������ͬ��2����ʱ�Թ����ռ�����H2�������___________mL��
(4)��������ʵ��ó����½��ۣ����в���ȷ����_________________��
A. ����Ħ�������������¶����
B. �ڸ�ʵ�������£�3 mol O2������Ħ�����Ϊ74.58 L/mol
C. ͬ�¡�ͬѹ�£�2 mol O2��2 mol CO��CO2�������������ͬ
D. ��ʵ�������£�O2���ܶ�Ϊ1.287 g/L
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com