
����Ŀ��A��B��C��D��E��ԭ���������ε��������ֳ���Ԫ�ء�A��BԪ����ɵ���̬������M��ˮ��Һ�ʼ��ԣ�CԪ���ǵؿ��к�������Ԫ�أ�D�ĵ�����C�ĵ�����ȼ�պ�IJ������ʹƷ����Һ��ɫ��E�ǽ���Ԫ�ء�
(1)д��A��C����Ԫ����ɵĻ�����A2C2�ĵ���ʽ_______��
(2)����E����Ͷ�������У�������dz��ɫ��Һ�Ρ���ε�������Һ��A2C2��Ӧ�����ӷ���ʽΪ______________________________________��
(3)R��B�������ͨ������³ʺ���ɫ������һ�Թ�R����ʹԪ��Bȫ��ת��Ϊ��������������Ӧ��ˮ����ʽ�������ʵ���������ʢ��R���Թܵ�����ˮ���У�______��
(4)��������Ѱ����ʵĴ����͵缫���ϣ���A2��B2Ϊ�缫��Ӧ���HCl��NH4Cl��ҺΪ�������Һ��������ȼ�ϵ�أ���д���õ�ص�������Ӧʽ_____________���ŵ�ʱ����Һ�е�H+����_________(��������)��
���𰸡�![]() 2Fe2+ + 2H+ +H2O2 = 2Fe3+ +2H2O ���Թ��л���ͨ���������� N2 +8H+ +6e- =2NH
2Fe2+ + 2H+ +H2O2 = 2Fe3+ +2H2O ���Թ��л���ͨ���������� N2 +8H+ +6e- =2NH![]() ����
����
��������
A��B��C��D��E��ԭ���������ε��������ֳ���Ԫ�ء�CԪ���ǵؿ��к�������Ԫ�أ���CΪOԪ�أ�A��B��ɵ���̬������M��ˮ��Һ�ʼ��ԣ�����֪BΪNԪ�ء�AΪHԪ�أ�MΪNH3��D�ĵ�����O2��ȼ�ղ����ʹƷ����Һ��ɫ������֪DΪSԪ�ء�
(1) A��C����Ԫ����ɵĻ�����A2C2ΪH2O2�����ڹ��ۻ���������ʽΪ![]() ��
��
(2) E�ǽ���Ԫ�أ���E����Ͷ�뵽������Һ�У�������dz��ɫ��ҺN����EΪFeԪ�أ�NΪFeCl2��Fe2+���л�ԭ�ԣ�H2O2���������ԣ��ʶ��߷�Ӧ�����ӷ���ʽΪ2Fe2+ + 2H+ +H2O2 = 2Fe3+ +2H2O��
(3)R��B�������ͨ������³ʺ���ɫ����RΪNO2������NO2ȫ��ת��ΪHNO3����ʢ��NO2���Թܵ�����ˮ���л������Թ��л���ͨ������������
(4) ��H2��N2Ϊ�缫��Ӧ���HCl��NH4Cl��ҺΪ�������Һ��������ȼ�ϵ�أ�����H2��N2�ϳɰ�ԭ����֪��H2ʧ���ӣ��Ӹ���ͨ�룬N2�õ��ӣ�������ͨ�룬��������ӦʽΪN2 +8H+ +6e- =2NH![]() ���ŵ�ʱ����Һ�е�H+Ϊ�����ӣ�Ӧ����������
���ŵ�ʱ����Һ�е�H+Ϊ�����ӣ�Ӧ����������


 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д� �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ����٤����ֵ�� ��֪HCl���������Ϊ3.65g
(1)HCl�����ʵ���Ϊ_______________
(2)HCl�ķ��Ӹ���Ϊ_______________
(3)ԭ������Ϊ _______________
(4)�ڱ�״�������Ϊ________________
(5)����������Ϊ _________________
(6)��� HCl��ȫ����ˮ���ó�1L ��Һ������Һ��H+�����ʵ���Ũ��Ϊ_________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��ش��������⣺
��1����A��ͬ�¡�ͬѹ���������ܶ���H2��35���������ʽΪ________��
��2��3������1һ��Ȳ�Ľṹ��ʽΪ________
��3��![]() �ķ���ʽΪ__________
�ķ���ʽΪ__________
��4������ʽΪC8H10���ڷ�������ͬ���칹�干��______�֣�����_______���ṹ��ʽ���ڱ����ϵ�һ��ȡ�����������֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��ӦA2+B2 = 2AB�������仯��ͼ��ʾ��������˵����ȷ����
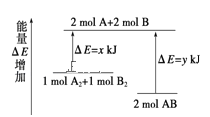
A.�÷�Ӧ�����ȷ�Ӧ
B.���� 1molA-A ���� 1molB-B ���ܷų�xkJ ������
C.2mol AB ������������1mol A2��1mol B2�͵�������
D.���� 2mol A-B ����Ҫ����ykJ ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ��Զδ��ȫ�����ľ�ѧ��Դ���⡣
(1)���辭����ѧ�仯���ܴӺ�ˮ�л�õ�������________������ţ�
A ���� B ��ˮ C�ռ� D ʳ��
(2)�Ӻ�ˮ�ƵõĴ����к��н϶��Mg2+��Ca2+��SO![]() �ȣ�Ҫ��ȥ��Щ���ӣ����м���ҩƷ˳����ȷ����________������ţ�
�ȣ�Ҫ��ȥ��Щ���ӣ����м���ҩƷ˳����ȷ����________������ţ�
A NaOH��Һ��Na2CO3��Һ��BaCl2��ҺB BaCl2��Һ��NaOH��Һ��Na2CO3��Һ
C NaOH��Һ��BaCl2��Һ��Na2CO3��ҺD Na2CO3��Һ ��NaOH��Һ�� BaCl2��Һ
(3)�Ӻ�ˮ�еõ���Ĺ������£�
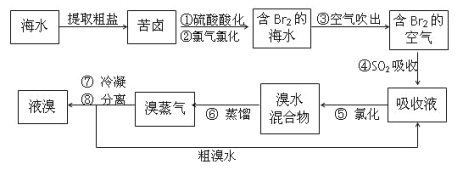
��д��������±����������庣ˮ�������ӷ���ʽ________
��д���ܷ�����Ӧ�Ļ�ѧ����ʽ________
��ij��ѧС���ͬѧΪ���˽�ӹ�ҵ�����ᴿ��ķ������������й����ϣ�Br2�ķе�Ϊ59 ��������ˮ���ж��Ժ�ǿ��ʴ�ԡ����Dzι��������̺����������ͼ��ʾʵ��װ����
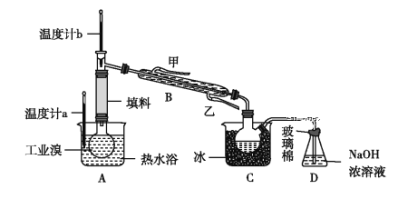
ͼ������B��ȴˮ�ij���Ϊ______(����������������)��Dװ�õ�������__________����Ӧ�����ӷ���ʽΪ__________������ʵ��װ�����������Ӿ�������������������ԭ����__________��
(4)��֪ij��Һ��Cl-��Br-��I-�����ʵ���֮��Ϊ2��3��4������ʹ��Һ�е�Cl-��Br-��I-�����ʵ���֮��Ϊ��Ϊ4��3��2����ôͨ��Cl2�����ʵ�����ԭ��Һ��I-�����ʵ�����__________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ܡ������仯�����ڹ�ҵ��ҽҩ����������ҪӦ�á��ش���������:
(1)�������Ͽ�ѧ����ʵ���ҿ���С�鷢������5K�³��ֳ����Եľ���CoO2���þ�����в�״�ṹ��
��������ԭ��Co��O����λ��֮��Ϊ_________��
����̬��ԭ�ӵļ۵����Ų�ͼΪ_______��
(2)�����Ni(CO)4������ΪҺ̬��������CCl4�������л��ܼ�����̬Ni(CO)4����_____���壻д��������CO������ͬ�ռ乹�ͺͼ�����ʽ�ķ��ӻ����ӣ�_______��
(3)ij�������ṹ��ͼ��ʾ:
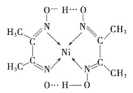
�������ں��еĻ�ѧ����___________(�����).
A ��� B ���Ӽ� C ���ۼ� D ������ E ��λ��
���������C��N��O����Ԫ��ԭ�ӵĵ�һ�������ɴ�С��˳����N> O>C���Դ�ԭ�ӽṹ����Ϊʲôͬ����Ԫ��ԭ�ӵĵ�һ������N>O_________��
(4)ij�о�С�齫ƽ���͵IJ��������ӽ��в�״������ʹÿ�������еIJ�ԭ����ijһ���������г��У������ܵ���������ӽ���" ����ṹ��ͼ��ʾ��

��"���ӽ���"���Ե��磬����Ϊ______���������еĽ���ԭ����������
��"���ӽ���"�У���ԭ���Ƿ���sp3�ķ�ʽ�ӻ�?_________(������"������")����������__________��
(5)�����������У���ԭ�ӵ���λ��Ϊ12��������������x��y��z���ͶӰͼ��ͼ��ʾ�������������ܶ�Ϊd g��cm-3��������a=_______nm(�м���ʽ)��
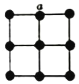
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��WΪ���ֶ���������Ԫ�أ�X��Y�����ڱ��е����λ����ͼ��ʾ��Xn��Yn+��Z+������ͬ�ĵ��Ӳ�ṹ��W�����ڲ������������������֮�͵��ڴ���������������˵����ȷ����

A. ԭ�Ӱ뾶��r(X)<r(Y)<r(Z)<r(W)
B. X�γɵ���������������Ϊ4��
C. ����������Ӧˮ����ļ��ԣ�Z<Y
D. Y��Z��W��Ӧ������������ˮ����֮���ܹ��������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʳ�ִ���������(A1P)Ѭ��ɱ�棬A1P��ˮ������ǿ��ԭ�Ե�PH3���塣���ұ��涨��ʳ������(��PH3��)�IJ�����������0.05 mgkg-1ʱΪ�ϸ�ijС��ͬѧ����ͼ��ʾʵ��װ�ú�ԭ���ⶨij��ʳ��Ʒ�е�����IJ�������C�м���100 gԭ����E �м���20.00mL2.50��lO-4molL-1KMnO4��Һ��H2SO4�ữ)��C�м�������ˮ����ַ�Ӧ�����������Ʊ���Һ�ζ�E�е���Һ��
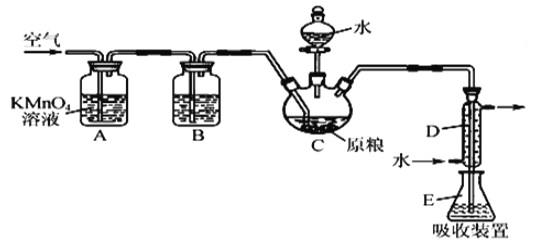
(1)װ��A�е�KMn04��Һ��������_____��
(2)װ��B��ʢװ����ûʳ����ļ�����Һ���տ����е�O2����ȥ����װ�ã����õ�����IJ�����___(����ƫ�{����ƫ��������������)��
(3)װ��E��PH3���������ᣬMnO4-����ԭΪMn2+��д���÷�Ӧ�����ӷ���ʽ��__________��
(4)�ռ�װ��E�е�����Һ����ˮϡ����250 mL����ȡ���е�25.00 mL����ƿ�У� ��4.0��lO-5molL-1��Na2SO3����Һ�ζ�������Na2SO3����Һ20.00mL����Ӧԭ���� S02-+Mn04-+H+��S042-+Mn2++H20(δ��ƽ)ͨ�������жϸ���Ʒ�Ƿ�ϸ�(д���������)_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ͼ��ʾװ�ý�����Ȫʵ���һ��������
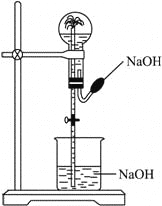
A.HCl��CO2B.NH3��CH4C.SO2��COD.NO2��NO
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com