
 2Fe(s)+3CO2(g) ��H =" a" kJ mol��1
2Fe(s)+3CO2(g) ��H =" a" kJ mol��1| | Fe2O3 | CO | Fe | CO2 |
| ��/mol | 1.0 | 1.0 | 1.0 | 1.0 |
| ��/mol | 1.0 | 2.0 | 1.0 | 1.0 |

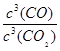 ��С
��С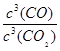 ����Ϊ�÷�ӦΪ���ȷ�Ӧ�������¶����ߣ�ƽ�������ƶ���Kֵ��С��
����Ϊ�÷�ӦΪ���ȷ�Ӧ�������¶����ߣ�ƽ�������ƶ���Kֵ��С��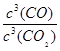 =
=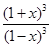 =64������õ�x=0.6����ת����=60%
=64������õ�x=0.6����ת����=60%


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ĵ�����������ȵ� |
| B����V��20ʱ����Һ�У�c��F������c��Na������0.1mol/L |
| C����V��20ʱ����Һ������Ũ�ȹ�ϵ����Ϊ��c��Na������c��F���� |
| D����V��20ʱ����Һ������Ũ�ȹ�ϵһ��Ϊ��c��Na������c��F������c��OH������c��H���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��(5a��2b) kJ | B��(2b��5a) kJ |
| C��(5a��2b) kJ | D��(10a��4b) kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 ��������Ӧ��Ӧ�Ļ�ѧ����ʽΪ ��
��������Ӧ��Ӧ�Ļ�ѧ����ʽΪ �� CH3OH(g) ��H1����90��7kJ��mol��1
CH3OH(g) ��H1����90��7kJ��mol��1 CH3OCH3(g)��H2O(g) ��H2����23��5kJ��mol��1
CH3OCH3(g)��H2O(g) ��H2����23��5kJ��mol��1 CO2(g)��H2(g) ��H3����41��2kJ��mol��1
CO2(g)��H2(g) ��H3����41��2kJ��mol��1| ���� | CH3OH | CH3OCH3 | H2O |
| c(mol/L) | 0��8 | 1��24 | 1��24 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 CH3OH(g)
CH3OH(g)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����H=1/2��H1 -��H2 | B����H=1/2��H1 +��H2 |
| C����H=1/2��H1 -1/2��H2 | D����H=1/2��H1 +1/2��H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH(g) ��H= ��90��8kJ/mol
CH3OH(g) ��H= ��90��8kJ/mol CH3OCH3(g)+H2O(g) ��H=��23��5kJ/mol
CH3OCH3(g)+H2O(g) ��H=��23��5kJ/mol CO2(g)+H2(g) ��H=��41��3kJ/mol
CO2(g)+H2(g) ��H=��41��3kJ/mol CH3OCH3(g)+CO2(g) �ġ�H= ��
CH3OCH3(g)+CO2(g) �ġ�H= �� CH3OCH3(g)+H2O(g) ��H=��23��5kJ/mol
CH3OCH3(g)+H2O(g) ��H=��23��5kJ/mol| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ�ȣ�mol��L-1�� | 0��40 | 0��6 | 0��6 |
 _________
_________ ���>������<����=������
���>������<����=�������鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com