
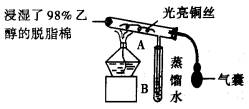
��ش��������⣺
(1)�����ȵ�ͭ˿��������Ӧ�Ļ�ѧ����ʽΪ_____________________________��
(2)��A���пɹ۲쵽��ʵ������Ϊ________________________________________���ɴ˿�����ʶ����ʵ������д����������ʱ�μ��˻�ѧ��Ӧ����������ʶ�������������ʱ��Ҫһ����____________________��
(3)ʵ��һ��ʱ�����������ƾ��ƣ���Ӧ__________(������ֹͣ)���У�ԭ���ȵ�ͭ˿����Ϊ__________ɫ��
(4)��֤�Ҵ���������Ļ�ѧ������________________________________________��
| (1)2Cu+O2 ��ʾ��  
��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д� ��ĩ1�����ʽ���������ϵ�д�
���ϰ��
��Ŀ�����л�ѧ ��Դ�� ���ͣ� �йش����Ĵ�������������Դӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ�ã��г�װ��������ʡ�ԣ�����ʵ�����Ϊ����ͼ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a��b��c��ͨ�����ƻ���a��b�����н��ࣨ��Ъ�ԣ�ͨ�����壬������M���۲쵽���Ե�ʵ�������Իش��������⣺  ��1��A�з�����Ӧ�Ļ�ѧ����ʽ�� 2H2O2
2H2O2 ��C����ˮ�����ã�
ʹD���Ҵ���Ϊ��������M�вμӷ�Ӧ ʹD���Ҵ���Ϊ��������M�вμӷ�Ӧ ����2��M�������ķ�Ӧ�Ļ�ѧ����ʽΪ�� 2CH3CH2OH+O2
2CH3CH2OH+O2 ��
��3����M���пɹ۲쵽������ ���Ȳ��ֵ�ͭ˿���ڼ�Ъ�Եع��������������ֱ�ڡ��������� ���Ȳ��ֵ�ͭ˿���ڼ�Ъ�Եع��������������ֱ�ڡ��������� ���п���ʶ����ʵ������д����μ� �μ� ����μӡ����μӡ����˻�ѧ��Ӧ����4����֤�Ҵ���������Ļ�ѧ��Ӧ����ʽ�ǣ� CH3CHO+2[Ag��NH3��2]OH
CH3CHO+2[Ag��NH3��2]OH ��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ����� ��2011?�Ϻ�ģ�⣩�йش����Ĵ�������������Դӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ�ã��г�װ��������ʡ�ԣ�����ʵ�����Ϊ���Ȱ�ͼ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a��b��c��ͨ�����ƻ���a��b�����н��ࣨ��Ъ�ԣ�ͨ�����壬������M���۲쵽���Ե�ʵ�������Իش��������⣺ 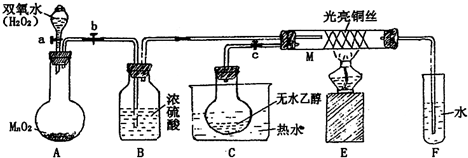 ��1��A�з�����Ӧ�Ļ�ѧ����ʽ�� 2H2O2
2H2O2 ��B�����ã�
����O2 ����O2 ��C����ˮ�����ã�C����ˮʹD���Ҵ���Ϊ��������M�вμӷ�Ӧ C����ˮʹD���Ҵ���Ϊ��������M�вμӷ�Ӧ ����2��M�������ķ�Ӧ�Ļ�ѧ����ʽΪ�� 2CH3CH2OH+O2
2CH3CH2OH+O2 ��
��3����M���пɹ۲쵽������ ���Ȳ��ֵ�ͭ˿���ڼ�Ъ�Եع��������������ֱ�ڣ��������� ���Ȳ��ֵ�ͭ˿���ڼ�Ъ�Եع��������������ֱ�ڣ��������� �����п���ʶ����ʵ������д����μ� �μ� ����μӡ����μӡ����˻�ѧ��Ӧ����������ʶ���������������Ҫһ�����¶� �¶� ����4����֤�Ҵ�����������Լ��� ����������ͭ����Һ ����������ͭ����Һ ����д����Ӧ�Ļ�ѧ����ʽCH3CHO+2Cu��OH��2
CH3CHO+2Cu��OH��2 ��
��5�����Թ�F���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л����� CH3COOH CH3COOH ��Ҫ��ȥ�����ʣ������ڻ��Һ�м���c c ����д��ĸ����a���Ȼ�����Һ������b�������� c��̼��������Һ ����d�����Ȼ�̼�� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2010-2011ѧ������ʡ�䶨һ�и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ���� ��7�֣��йش����Ĵ������������ǿ��Դӡ��Ҵ���ʵ�顱�еõ�һЩ��ʶ��ij��ʦ�������ͼ��ʾװ�ã��г�װ����ʡ�ԣ����Իش��������⣺ �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2014�������ʡ�߶���ѧ�ڵ�һ�μ�⻯ѧ�Ծ��������棩 ���ͣ������ �йش����Ĵ�������������Դӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ�ã��г�װ��������ʡ�ԣ�����ʵ�����Ϊ����ͼ��װ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a��b��c��ͨ�����ƻ���a��b�����н��ࣨ��Ъ�ԣ�ͨ�����壬������M���۲쵽���Ե�ʵ������
��1��A�з�����Ӧ�Ļ�ѧ����ʽ��_____________��B�����ã�____________�� C�����ã�____________�� ��2��M�������ķ�Ӧ�Ļ�ѧ����ʽΪ��______________________________________ ��3����M���пɹ۲쵽������Ϊ_______________�����п���ʶ����ʵ������д���___ __ ____����μӡ����μӡ����˻�ѧ��Ӧ ��4����֤�Ҵ�����������Լ��� ����д����Ӧ�Ļ�ѧ����ʽ �� ��5�����Թ�F���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л����� ��Ҫ��ȥ�����ʣ������ڻ��Һ�м��� ����д��ĸ���� A���Ȼ�����Һ B���� C��̼��������Һ D�����Ȼ�̼
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2012������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ���� ��7�֣��йش����Ĵ������������ǿ��Դӡ��Ҵ���ʵ�顱�еõ�һЩ��ʶ��ij��ʦ�������ͼ��ʾװ�ã��г�װ����ʡ�ԣ����Իش��������⣺
��1��A�з�����Ӧ�Ļ�ѧ����ʽΪ��___________________________ �� ��2����Ӧһ��ʱ�������ȥ�ƾ��Ʒ�Ӧ���ܼ������У���˵�����Ҵ���������Ӧ��_________________(����ȡ������ȡ�)��Ӧ�� ��3��ʵ�������D��ͭ˿���к�ɫ�ͺ�ɫ������ֵ��������û�ѧ����ʽ����ԭ������٣���ɫ���ɫ��___________________________________________�� ����ڣ���ɫ���ɫ��____________________________________________ ����Щʵ�������п�����ʶ��ʵ������д���__________����μӡ����μӡ�����ѧ��Ӧ�� ��4��װ��B��F�����÷ֱ��� B:______________________________________ , F:_________________________________ ��
�鿴�𰸺ͽ���>> ͬ����ϰ��� ����ѧУ��ѡ - ��ϰ���б� - �����б� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||