
����Ŀ��I.��1��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
�ټ��������Ը��������Һ����úȼ�ղ�����SO2���ù����и����������ԭΪMn2+����д���ù��̵����ӷ���ʽ______________��
�ڽ�ȼú�����Ķ�����̼���Ի��գ��ɽ���̼���ŷš���ͼ��ͨ���˹�������ã���CO2��H2OΪԭ���Ʊ�HCOOH��O2��ԭ��ʾ��ͼ��a�缫���ƣ�_____________(�������������)��b�缫�ķ�Ӧʽ��________________________��

��2���������NaClO��Ca��ClO��2�����ռ���Ҳ�ܵõ��Ϻõ���������Ч����
��֪���з�Ӧ��
SO2(g)+2OH��(aq) ==SO32��(aq)+H2O(l) ��H1
ClO��(aq)+SO32��(aq) ==SO42��(aq)+Cl��(aq) ��H2
CaSO4(s)==Ca2+��aq��+SO42����aq�� ��H3
��ӦSO2(g)+ Ca2+��aq��+ ClO��(aq) +2OH��(aq) = CaSO4(s) +H2O(l) +Cl��(aq)�Ħ�H=_____��
II.��3��FeO42����ˮ��Һ�еĴ�����̬��ͼ��ʾ��

������pH��10��������Һ�м�������pH��2��HFeO4���ķֲ������ı仯�����__________��
������pH��6��������Һ�еμ�KOH��Һ������Һ�к���Ԫ�ص����У�_________ת��Ϊ_________(��������)��
���𰸡�2MnO4��+5SO2+2H2O=2Mn2++5SO42��+4H+ ����CO2+2e��+2H+=HCOOH��H1+��H2-��H3 ��������СHFeO4��FeO42��
��������
(1)�������Ը��������Һ����úȼ�ղ�����SO2�������и����������ԭΪMn2������SO2������ΪSO42�����ݴ�д����Ӧ�����ӷ���ʽ��
��a�缫����H2Oת��ΪO2���˹���Ϊʧȥ���ӵķ�Ӧ��ԭ�����ʧȥ���ӵĵ缫Ϊ������b�缫Ϊ������CO2��H���õ����ӣ�����HCOOH��
(2)���ݸ�˹���ɽ��⡣
��3���ٸ���ͼƬ֪�����ݲ�ͬpHʱ��HFeO4���ı仯ͼ���жϣ�����pH=6��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��HFeO4��+OH��=FeO42��+H2O��
(1)���������Ը��������Һ����úȼ�ղ�����SO2�������и����������ԭΪMn2������SO2������ΪSO42������ù��̵����ӷ���ʽΪ��5SO2+2MnO4��+2H2O�T2Mn2��+5SO42��+4H����
��a�缫����H2Oת��ΪO2���˹���Ϊʧȥ���ӵķ�Ӧ��ԭ�����ʧȥ���ӵĵ缫Ϊ������b�缫Ϊ������CO2��H���õ����ӣ�����HCOOH����b�缫�ķ�Ӧʽ��CO2+2H��+2e���THCOOH
��2�����ݸ�˹���ɣ���SO2(g)+2OH��(aq) ==SO32��(aq)+H2O(l) ��H1
��ClO��(aq)+SO32��(aq) ==SO42��(aq)+Cl��(aq) ��H2
��CaSO4(s)==Ca2+��aq��+SO42����aq�� ��H3
���+��-�۵÷�ӦSO2(g)+ Ca2+��aq��+ ClO��(aq) +2OH��(aq) = CaSO4(s) +H2O(l) +Cl��(aq)�Ħ�H= ��H1+��H2-��H3 ��
��3����ͼ�������֪������pH��10��������Һ�м�������pH��2��HFeO4���ķֲ������ı仯�������������С������pH=6��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪ��HFeO4��+OH��=FeO42��+H2O��HFeO4��ת��ΪFeO42����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��ɫ��Һ�����п��ܺ���Fe3+��Al3+��Fe2+��Mg2+��Cu2+��NH4����K+��CO32����SO42�������ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й�������ͼ��ʾ��

���������ͼ�ƶ���
��1��ԭ��Һ��һ�����ڵ���������_______________����___(����������������������)�ԡ�
��2��ʵ����в�����ɫ��ζ�����������Ļ�ѧ����ʽΪ__________________________________��
��3��д��ʵ�����A���Ӧ�����Ļ�ѧʽ��__________��
��4��д��ʵ���������A��B��������������Ӧ�����ӷ���ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������H��һ�ַ����߾���(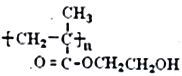 )�ĵ��塣�ɻ�����A(C4H8)�Ʊ�H��һ�ֺϳ�·��������
)�ĵ��塣�ɻ�����A(C4H8)�Ʊ�H��һ�ֺϳ�·��������
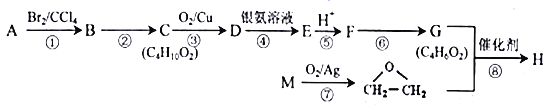
��֪��A��M��Ϊͬϵ��ش��������⣺
��1��A��ϵͳ����Ϊ_____________��D�����к��еĹ���������Ϊ__________________��
��2��F��G�ķ�Ӧ����Ϊ______________________����Ӧ�������ķ�Ӧ���ͷֱ�Ϊ_________��_________��
��3����Ӧ���Ļ�ѧ����ʽΪ___________________________________��
��4��������XΪH��ͬ���칹�壬X�������Ƶ�������ͭ����Һ��Ӧ����ש��ɫ������������Na2CO3������Һ��Ӧ�ų����壬��˴Ź���������4�ַ塣д�����ַ���Ҫ���X�Ľṹ��ʽ_____________________________________________________��
��5�����������ϳ��е���Ϣ������д������ϩ������Ϊԭ�Ͼ������Ʊ�CH3-COOCH=CH2�ĺϳ�·��_______________________________________(�����Լ���ѡ���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����(����)
A. �ð״׳����⣺Fe2O3��6H��==3H2O��2Fe3��
B. ��NH4HCO3��Һ�м��������Ba(OH)2��Һ�����ȣ�Ba2����2OH����NH4����HCO3��![]() NH3����2H2O��BaCO3��
NH3����2H2O��BaCO3��
C. �Ȼ�������Һ�ڿ������������������ˮ��������ɫ������Fe2++2NH3H2O��Fe(OH)2��+2NH4+
D. NH4HS��Һ��������NaOH��Һ��Ӧ��NH4++OH��=NH3��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ������������й������������(����)
A. һ�������£�2molSO2��1molO2������ܱ������г�ַ�Ӧ�������еķ���������2NA
B. 1 mol Na��O2��ȫ��Ӧ������Na2O��Na2O2�Ļ���ת�Ƶ�������ΪNA��
C. 16 g CH4��18 g NH4+ ������������Ϊ10NA
D. ��Һ�к��Ȼ���1mol����ȫת��Ϊ�����������������ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵ�ԭ������������������AԪ��ԭ�ӵĺ���p��������s��������1��C�ǵ縺������Ԫ�ء�Dԭ�Ӵ���������������������2����E�ǵڢ�����ԭ��������С��Ԫ�ء�
��1��д����̬Cԭ�ӵĵ����Ų�ʽ_________________��
��2��A��B��C����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ______________(��Ԫ�ط��ű�ʾ)��ԭ����___________________��
��3����֪DC4������Ϊ���壬������ʵľ���������_________�������������ԭ�ӵĹ���ӻ�����Ϊ____________���ռ乹����___________��
��4��Cu2��������AH3�γ�������[Cu(AH3)4]2������AC3������Cu2���γ������ӣ���ԭ����______________________��
��5��A��B��Ԫ�طֱ���D�γɵĹ��ۼ��У����Խ�ǿ����__________��A��B��Ԫ�ؼ����γɶ��ֶ�Ԫ�����������A3����Ϊ�ȵ���������ʵĻ�ѧʽΪ__________��
��6����֪E���ʵľ�����ͼ��ʾ��������Eԭ�ӵ���λ��Ϊ__________��һ��E�ľ�������Ϊ___________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ȼ�������(�ṹ��ʽΪ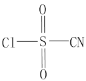 )��һ�ֶ���;���л��ϳ��Լ�����HClO4-NaClO4�������� K5[Co3+O4W12O36](��дΪCo3+W)�ɴ��ϳ��Ȼ���������
)��һ�ֶ���;���л��ϳ��Լ�����HClO4-NaClO4�������� K5[Co3+O4W12O36](��дΪCo3+W)�ɴ��ϳ��Ȼ���������
��1����̬��ԭ�ӵĺ�������Ų�ʽΪ__________�����HClO4-NaClO4��4��Ԫ�صĵ縺����С�����˳��Ϊ__________
��2���Ȼ���������������ԭ�Ӻ�̼ԭ�ӵ��ӻ�������ͷֱ���__________��__________�� 1���Ȼ������������к�����������ĿΪ__________���Ȼ���������5��Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ__________��
��3��ClO4-�Ŀռ乹��Ϊ__________
��4��һ��������̼�γɵļ�϶������ľ���ṹ��ͼ��ʾ������̼ԭ��λ����ԭ���γɵİ������������ÿ����ԭ����Ϊ���������干������û�����Ļ�ѧʽΪ__________
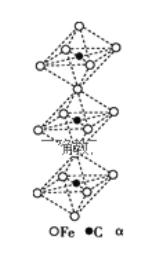
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��500 mL KNO3��Cu(NO3)2�Ļ����Һ��c(N)= 6��0 mol��L-1����ʯī���缫������Һ����ͨ��һ��ʱ����������ռ���22��4 L����(��״��)���ٶ�������Һ�����Ϊ500 mL������˵����ȷ����( )
A. ԭ�����Һ��c(K+)Ϊ2 mol��L-1
B. �����������й�ת��2 mol����
C. ���õ���Cu�����ʵ���Ϊ0��5 mol
D. ������Һ��c(H+)Ϊ2 mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ͼ��ʾװ��(�̶�����������������)�����йذ���ȡ��ʵ��̽�����ش��������⣺
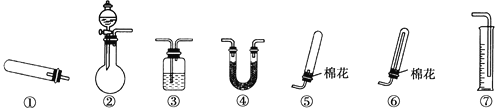
(1)����װ�â���ȡNH3���䷴Ӧ�Ļ�ѧ����ʽΪ__________����Ҫ�ⶨ���ɵ�NH3������������ѡ���װ����________(��װ�����)��װ������ʢ�Լ�Ӧ���е�������________��
(2)����װ�â���ȡ���ռ������NH3����ƿ��װ���Լ�������__����Һ©����װ���Լ�������________���ռ�װ��Ӧѡ��________(��װ�����)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com