
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

|
�鿴�𰸺ͽ���>>
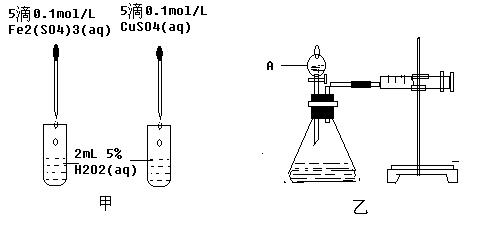
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| | ����ʽ | ��ɫ��״̬ | ˮ���� | �۵�/�� | �ܶ�/g��cm��3 |
| ���ᾧ�� | H2C2O4��2H2O | ��ɫ���� | ������ˮ | 101.5 | 1.650 |
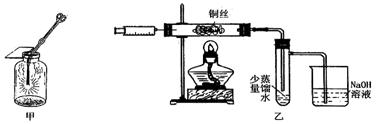
 ��2���������ɺ������Թ��е�����ˮ��Ϊ����ɫ����ʱ��������������ʹ����NaOH��Һ�������Թ��У������Թ����� ɫ�ij����������÷�Ӧ���뷽��ʽΪ ��
��2���������ɺ������Թ��е�����ˮ��Ϊ����ɫ����ʱ��������������ʹ����NaOH��Һ�������Թ��У������Թ����� ɫ�ij����������÷�Ӧ���뷽��ʽΪ ���鿴�𰸺ͽ���>>
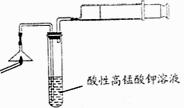
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
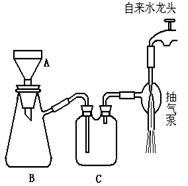
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ���� | ʵ����� | Ԥ������ |
| ����һ��FeԪ��ֻ��______�� | ����������Һ�е���KSCN��Һ ����ϡ����KMnO4��Һ�е���������Һ | KSCN��Һ���������� |
| �����;FeԪ��ֻ��______�� | ϡ����KMnO4��Һ��ɫ_____ | |
| ��������FeԪ�ؼ���+2������+3�� | KSCN��Һ��______ɫ ϡ����KMnO4��Һ��ɫ______ |
�鿴�𰸺ͽ���>>
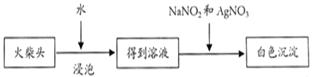
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

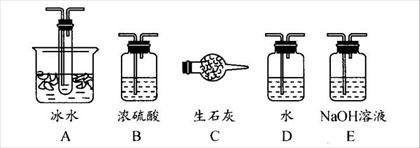
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ƺ�þ�ֱ�����ˮ��Ӧ���ж��ƺ�þ������ǿ�� |
| B����MgCl2��AlCl3��Һ�зֱ��������İ�ˮ���ж�þ�����Ľ�����ǿ�� |
| C����������Һ��ͨ��CO2������ɫ�������ж�̼������������ǿ�� |
| D��Br2��I2�ֱ���������H2��Ӧ���ж������ķǽ�����ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������Ȼ�þ��ȡ����þ |
| B�������ƽᾧˮ�������ʯ�ҹ�����ȡ���顣 |
| C��Ũ�������廯�ƹ�����ȡ�廯�� |
| D������̼������Һ��ȥ������̼�е��Ȼ��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com