
��. ���Т�0.2mol/L NaOH��Һ�͢�0.2mol/L HX��Һ������Һ�������Ϻ����Һ�� c(Na+)>c(X-)��
(1) ���Һ�� �ԣ���ᡱ��������С�������ԭ�������ӷ���ʽ��ʾ
��
��2) �������Һ�У����>������=�硱��<����
c(Hx) c(X-)�� c(OH-) c(HX)+c(H+��
����ͼ��һ���ò�˿���缫�����ϡ��MgS04��Һ��װ�ã����Һ�е��з�̪�Լ����ش�
�������⣻
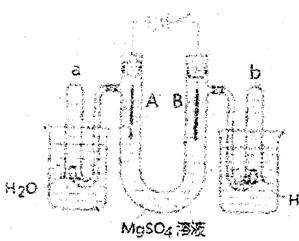
(1) ͨ��ʱ��A���е������� ��
(2) ��ͨ��ʱ��A���з����ķ�Ӧ����ʽ�� ��
��ͨ��ʱ��B���з����ķ�Ӧ����ʽ�� ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| m |
| M |
| m |
| M |
| 2m?NA |
| M |
| 2m?NA |
| M |
| 22.4m |
| M |
| 22.4m |
| M |
| m |
| M?L |
| m |
| M?L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | ��ʼʱ�����ʵ��� | ����ƽ��ʱ�ų���������QkJ�� | ƽ��ʱSO2ת���� ��X�� | |||
| SO2��mol�� | O2��mol�� | SO3��mol�� | N2 | |||
| �� | 2 | 1 | 0 | 0 | Q1 | X1 |
| �� | 1 | 0.5 | 0 | 0 | Q2=39.4 | X2 |
| �� | 1 | 0.5 | 0 | 1 | Q3 | X3 |
| �� | 1.8 | 0.9 | 0.2 | 0 | Q4 | X4 |

| t1-t2 | t3-t4 | t4-t5 | t6-t7 |
| K1 | K2 | K3 | K4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ͼ������ͼ���dz��õĿ�ѧ�о�������
ͼ������ͼ���dz��õĿ�ѧ�о�������| Ԫ �� | I1/eV | I2/eV | I3/eV |
| L | 13.0 | 23.9 | 40.0 |
| M | 4.3 | 31.9 | 47.8 |
| N | 5.7 | 47.4 | 71.8 |
| O | 7.7 | 15.1 | 80.3 |
| P | 21.6 | 41.1 | 65.2 |
| 13 |
| 16 |
| 13 |
| 16 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ������һ�и�����һ��������⻯ѧ�Ծ����������� ���ͣ�������
�����dz���������ǿ��֮һ���ڻ�ѧ�о��ͻ������������Ź㷺Ӧ�ã��������Ʊ������Ρ�Ⱦ�ϡ����ϡ�ҽҩ�м��塢����ըҩ�ȡ������ζ���������Լ���ͼ������ҵ��
��1��ij����M������������ʱ����ʽ�ֽ⣺2MNO3 2M+2NO2��+O2��������3.40gMNO3������NO2��O2����ɱ�״��ʱ�������Ϊ672mL���ɴ˿��Լ����M�����ԭ������Ϊ_____________��
2M+2NO2��+O2��������3.40gMNO3������NO2��O2����ɱ�״��ʱ�������Ϊ672mL���ɴ˿��Լ����M�����ԭ������Ϊ_____________��
��2����32.64gͭ��140mL һ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ������NO��NO2�����������ɱ�״���µ����Ϊ11.2L������NO�����Ϊ_____________��
��3������Cu��Cu2O��CuO��ɵĻ���ij�о���ѧϰС��Ϊ��̽����������������100mL0.6mol/LHNO3��Һǡ��ʹ�������ȫ�ܽ⣬ͬʱ�ռ���224mLNO���壨��״�����������������ͭ�����ʵ���Ϊ ����ԭ���������0.0lmolCu��������Cu2O��CuO��������Ϊ_____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com