
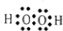 ��H2O2��HΪ+1�ۣ��ɻ��������������ϼ۵Ĵ�����Ϊ0������Ԫ�صĻ��ϼ�Ϊ-1�ۣ�
��H2O2��HΪ+1�ۣ��ɻ��������������ϼ۵Ĵ�����Ϊ0������Ԫ�صĻ��ϼ�Ϊ-1�ۣ�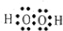 ��-1��
��-1�� 2H2O+O2�����ʴ�Ϊ��2H2O2
2H2O+O2�����ʴ�Ϊ��2H2O2 2H2O+O2����
2H2O+O2����

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2009��3���𣬴�ī���硢�����ȹ�����ɢ��ȫ����ļ���H1N1�����б������飬������ȫ���ע�ͻ���Ӧ�ԡ�����ר�ұ�ʾ���������������������ǿ�������������ɷ�����H1N1���С�
��1����̼������һ���ж���;��������ϵ��̬Ư������ѧʽ�ɱ�ʾΪNa2CO3��3H2O2��������Na2CO3��H2O2��˫�����ʡ���H2O2��ʱ����Ϊ��ҵ��Һ���������С���ɫ�������������ƣ��������ɿ�ҵ��Һ�е��軯���KCN���������·�Ӧʵ�֣�KCN+H2O2+H2O===A+NH3������������A�Ļ�ѧʽΪ ��H2O2����Ϊ����ɫ���������������� ����ijǿ���Է�Ӧ��ϵ�У���Ӧ��������ﹲ�������ʣ�O2��MnO-4��H2O��Mn2+��H2O2��H+����֪�÷�Ӧ��H2O2ֻ���������¹��̣�H2O2��O2��д���÷�Ӧ�����ӷ���ʽ��������ƽ���� ��
��2��Ư���������ƣ�NaClO2���ڳ�����ڰ����ɱ���һ�ꡣ������ȶ��ɷֽ⣬��Ӧ�����ӷ���ʽΪ��HClO2��ClO2��+H++Cl-+H2O��δ��ƽ�����ڸ÷�Ӧ�У�����1molClO2����ʱת�Ƶĵ��Ӹ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ������2010�������һ��ģ�⿼�Ի�ѧ�Ծ� ���ͣ���ѡ��
��7�֣���2009��3���𣬴�ī���硢�����ȹ�����ɢ��ȫ����ļ���H1N1�����б������飬������ȫ���ע�ͻ���Ӧ�ԡ�����ר�ұ�ʾ���������������������ǿ�������������ɷ�����H1N1���С�
��1����̼������һ���ж���;��������ϵ��̬Ư������ѧʽ�ɱ�ʾΪNa2CO3��3H2O2��������Na2CO3��H2O2��˫�����ʡ���H2O2��ʱ����Ϊ��ҵ��Һ���������С���ɫ�������������ƣ��������ɿ�ҵ��Һ�е��軯���KCN���������·�Ӧʵ�֣�KCN+H2O2+H2O===A+NH3������������A�Ļ�ѧʽΪ ��H2O2����Ϊ����ɫ���������������� ����ijǿ���Է�Ӧ��ϵ�У���Ӧ��������ﹲ�������ʣ�O2��MnO-4��H2O��Mn2+��H2O2��H+����֪�÷�Ӧ��H2O2ֻ���������¹��̣�H2O2��O2��д���÷�Ӧ�����ӷ���ʽ��������ƽ���� ��
��2��Ư���������ƣ�NaClO2���ڳ�����ڰ����ɱ���һ�ꡣ������ȶ��ɷֽ⣬��Ӧ�����ӷ���ʽΪ��HClO2��ClO2��+H++Cl-+H2O��δ��ƽ�����ڸ÷�Ӧ�У�����1molClO2����ʱת�Ƶĵ��Ӹ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2009��3���𣬴�ī���硢�����ȹ�����ɢ��ȫ����ļ���H1N1�����б������飬������ȫ���ע�ͻ���Ӧ�ԡ�����ר�ұ�ʾ���������������������ǿ�������������ɷ�����H1N1���С�
��1����̼������һ���ж���;��������ϵ��̬Ư������ѧʽ�ɱ�ʾΪ
Na2CO3��3H2O2��������Na2CO3��H2O2��˫�����ʡ�
��H2O2��ʱ����Ϊ��ҵ��Һ���������С���ɫ�������������ƣ��������ɿ�ҵ��Һ�е��軯���KCN���������·�Ӧʵ�֣�KCN+H2O2+H2O===A+NH3������������A�Ļ�ѧʽΪ ��H2O2����Ϊ����ɫ���������������� ��
��ijǿ���Է�Ӧ��ϵ�У���Ӧ��������ﹲ�������ʣ�O2��MnO-4��H2O��Mn2+��H2O2��H+����֪�÷�Ӧ��H2O2ֻ���������¹��̣�H2O2��O2��д���÷�Ӧ�����ӷ���ʽ��������ƽ���� ��
��2��Ư���������ƣ�NaClO2���ڳ�����ڰ����ɱ���һ�ꡣ������ȶ��ɷֽ⣬��Ӧ�����ӷ���ʽΪ��HClO2��ClO2��+H++Cl-+H2O��δ��ƽ�����ڸ÷�Ӧ�У�����1molClO2����ʱת�Ƶĵ��Ӹ����� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com