
 ����д���������ĸ���ϵʽ�������жϣ�����ȷ�Ĺ�ϵʽ�����
����д���������ĸ���ϵʽ�������жϣ�����ȷ�Ĺ�ϵʽ����� �����ڴ���ĺ���д����ȷ�Ĺ�ϵʽ��
�����ڴ���ĺ���д����ȷ�Ĺ�ϵʽ�� HCO3��+OH��(2��)
HCO3��+OH��(2��) (1��)
(1��) (1��)
(1��)


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��Ҫ�ɷ�������������������������ˮ������
��Ҫ�ɷ�������������������������ˮ������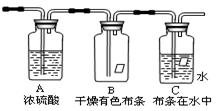
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 CO2����2H2O��2SO2��
CO2����2H2O��2SO2��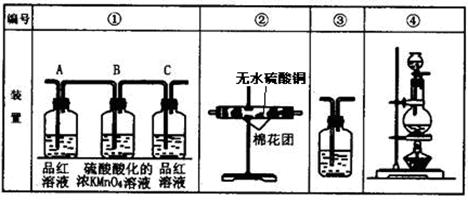
 ��˳���������������ҵķ����ǣ���װ�õı�ţ���
��˳���������������ҵķ����ǣ���װ�õı�ţ��� �����ܡ���__________��__________��__________��
�����ܡ���__________��__________��__________��| A��BaCl2��Һ | B��Ba(OH)2��Һ |
| C���μ�H2O2��BaCl2��Һ | D���μ�H2O2��Ba(OH)2��Һ |
 ��������Ϊbg����a��b��ʾCO2��SO2�����ʵ���������������������
��������Ϊbg����a��b��ʾCO2��SO2�����ʵ����������������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
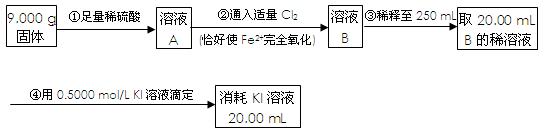
 ���ԭϡ��Һ��I-Ũ�ȣ��ζ�ʱ�ķ�ӦΪ��2Na2S2O3 + I2 = Na2S4O6 + 2NaI���Իش�
���ԭϡ��Һ��I-Ũ�ȣ��ζ�ʱ�ķ�ӦΪ��2Na2S2O3 + I2 = Na2S4O6 + 2NaI���Իش� ____________����ǡ����ǡ�������ΪAgX�����������Ļ�ѧʽΪ__________������ΪAgX��������˿ղ����
____________����ǡ����ǡ�������ΪAgX�����������Ļ�ѧʽΪ__________������ΪAgX��������˿ղ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������C2H5Cl�е���Ԫ��ʱ����C2H5Cl��NaOH��Һ��ϼ��Ⱥ���ϡ���������ữ���ټ���������Һ |
| B������Һ�м������ᣬ����ɫ�����ݳ�����������ʹ����ʯ��ˮ����ǣ������Һ�к���CO32- |
| C����0.1mol��L��1 FeSO4��Һ�еμ���������KMnO4��Һ��KMnO4��Һ��ɫ��˵��Fe2+���������� |
| D����2.0mLŨ�Ⱦ�Ϊ0.1mol��L��1��KCl��KI�����Һ�еμ�1~2��0.01mol��L��1 AgNO3��Һ���������ʻ�ɫ��˵��AgCl ��Ksp��AgI ��Ksp�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
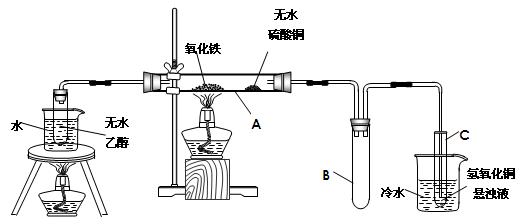
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ��Ŀ�� | ʵ����� |
| A | ʵ�����Ʊ������� | ������Ũ�����Ϻ���� |
| B | ��ȥ��������Һ�������Ȼ������� | �����Ȼ������ʵ���������Һ�м�����������������Һ������ |
| C | ���������鷢����ȥ��Ӧ�IJ��� | ��ʢ��������������Թ��У��ȼ�������������Һ�����ȣ��ٵ����������ữ ����������Һ ����������Һ |
| D | Ũ�����������ǿ��ϡ���� | ��ʢ��������Ũ���ᡢϡ�������֧�Թ��У��ֱ�����С��ͬ��ͭƬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | ��ij����ͨ��Ʒ����Һ�У���Һ��ɫ | ����������SO2 |
| B | �����£��ֱ���2֧�Թ��м�����ͬ�������ͬŨ�ȵ�Na2S2O3��Һ���ٷֱ������ͬ�����ͬŨ�ȵ�ϡ���� | �о�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�� |
| C | �²�Һ��ӷ�Һ©���¶˹ܿڷų��رջ�������һ�������������ٽ��ϲ�Һ����¶˹ܿڷų� | ȡ����Һ©���е��ϲ�Һ�� |
| D | ��ij��Һ�м���2��KSCN��Һ����Һ���Ժ�ɫ��������Һ�м��뼸�����Ƶ���ˮ����Һ��Ϊ��ɫ | ˵������Һ��һ������Fe2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ʵ�Ԫ����ɣ����������Ϊ���ʺͻ����� |
| B��ͨ��þ��п�����ֱ���ϡ���ᷴӦ��ʵ�����Ƚϲ�ͬ�����Ļ�����ǿ�� |
| C����������Ʒ����������ϡ�����ܽ��ȥ����ʵ��˵��������Ҫ�ɷ������������� |
| D����ʢװŨ������Լ�ƿ�����۲������˽������ijЩ�������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com